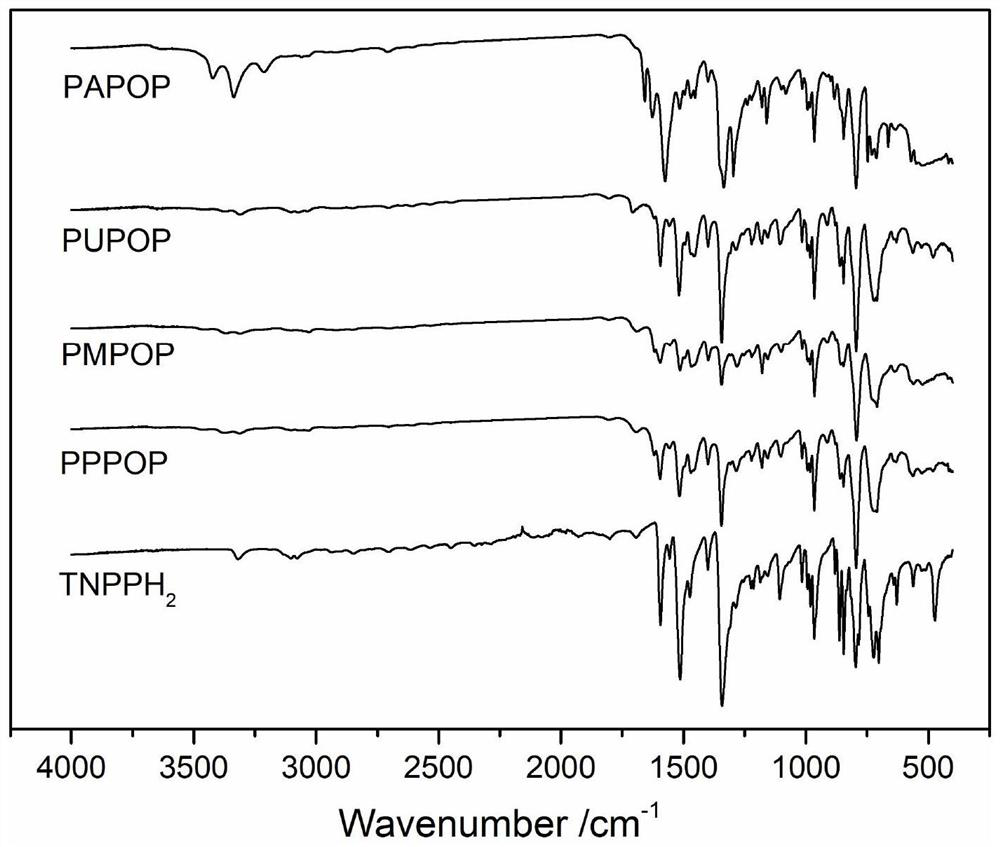
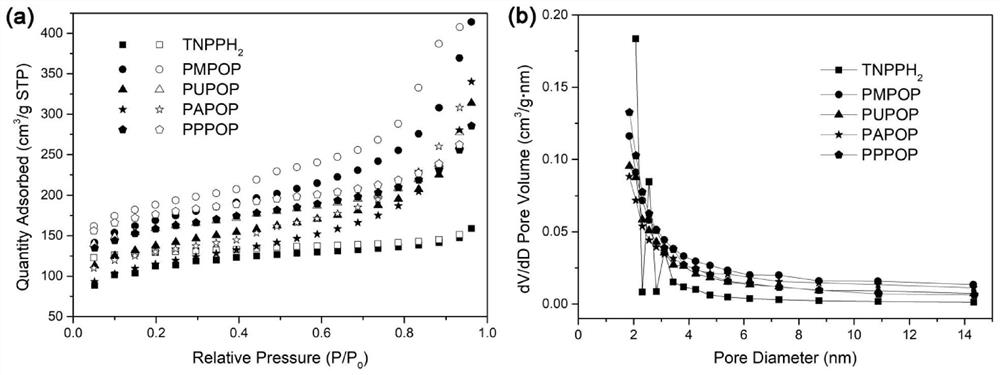
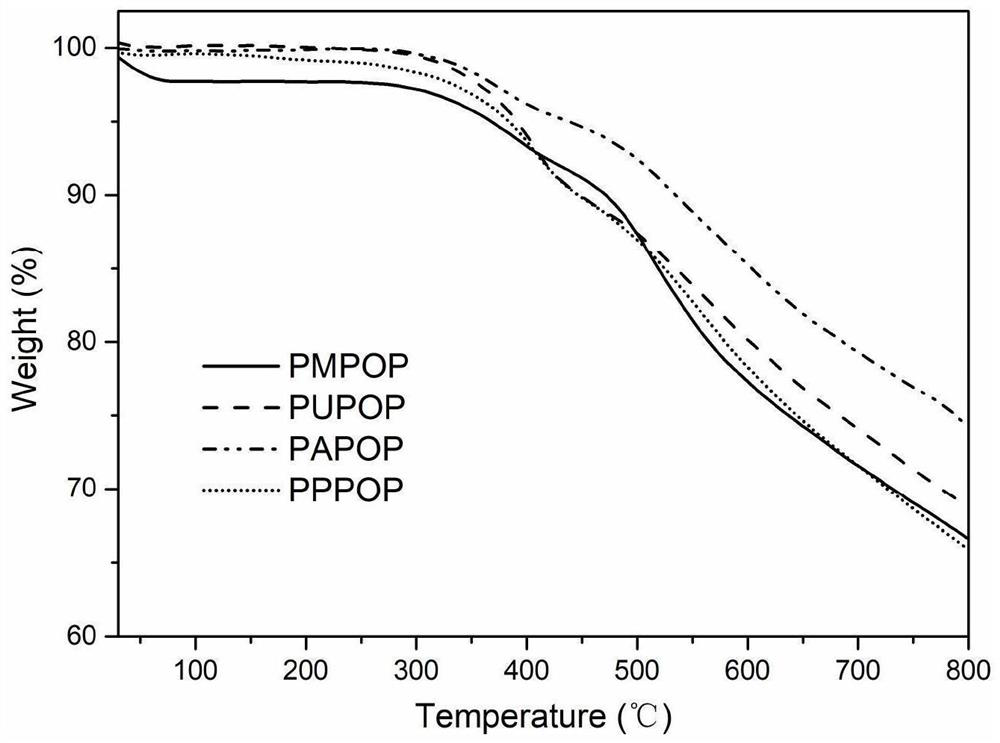
Porphyrin-based porous organic polymer, preparation method thereof, and preparation method and application of supported palladium catalyst of porphyrin-based porous organic polymer
A palladium catalyst and polymer technology, applied in the field of porphyrin-based porous organic polymers, can solve problems such as the need for additional reducing agents, harsh reaction conditions, and complicated preparation processes, and achieve high catalytic activity and selectivity, and mild reaction conditions , the effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Pd-PMPOP(H 2 PdCl 4 ) (using chloropalladium acid as the palladium source, the theoretical loading of palladium is 10wt%): take by weighing the porphyrin-based porous polymer PMPOP (48mg), add 20ml of deionized water, and ultrasonically disperse for 1h; then slowly drip 5mM chloropalladium Acid solution; filter after continuous stirring for 72 hours, and wash three times with deionized water and ethanol, and obtain the catalyst after drying. After testing, the actual content of palladium in the catalyst was 9.414 wt %.
[0061] Pd-PMPOP(PdCl 2 ) (using palladium dichloride as palladium source, the theoretical loading of palladium is 10wt%): Weigh PMPOP (54mg) and palladium dichloride (10mg) and add ethanol 10mL, namely m (carrier): m (palladium)= 9:1, ultrasonic for 3 hours, then centrifuged, alternately washed three times with deionized water and 50% ethanol, and dried to obtain the catalyst. After testing, the actual content of palladium in the catalyst is 9.365wt...
Embodiment 2
[0064] Pd-PUPOP(H 2 PdCl 4 ) (with chloropalladium acid as palladium source, the theoretical loading of palladium is 10wt%): take by weighing porous polymer PUPOP (48mg), add 20ml of deionized water, ultrasonically disperse 1h; then slowly drip the chloropalladium acid solution of 5mM; After continuous stirring for 72 hours, the mixture was filtered, washed three times with deionized water and ethanol, and dried to obtain the catalyst. After testing, the actual content of palladium in the catalyst was 9.342 wt %.
[0065] Pd-PUPOP(PdCl 2 ) (using palladium dichloride as palladium source, the theoretical loading of palladium is 10wt%): Weigh PUPOP (54mg) and palladium dichloride (10mg) and add ethanol 10mL, namely m (carrier): m (palladium)= 9:1, ultrasonic for 3 hours, then centrifuged, alternately washed three times with deionized water and 50% ethanol, and dried to obtain the catalyst. After testing, the actual content of palladium in the catalyst was 9.935wt%.
[0066]...
Embodiment 3
[0068] Pd-PAPOP(H 2 PdCl 4 ) (with chloropalladium acid as palladium source, the theoretical loading of palladium is 10wt%): take porous polymer PAPOP (48mg), add 20ml of deionized water, ultrasonically disperse for 1h; then slowly drip the 5mM chloropalladium acid solution; After continuous stirring for 72 hours, the mixture was filtered, washed three times with deionized water and ethanol, and dried to obtain the catalyst. After testing, the actual content of palladium in the catalyst was 7.995wt%.
[0069] Pd-PAPOP(PdCl 2 ) (using palladium dichloride as the palladium source, the theoretical loading of palladium is 10wt%): Weigh PAPOP (54mg) and palladium dichloride (10mg) and add 10mL of ethanol, namely m (carrier): m (palladium)= 9:1, ultrasonic for 3 hours, then centrifuged, alternately washed three times with deionized water and 50% ethanol, and dried to obtain the catalyst. After testing, the actual content of palladium in the catalyst was 9.666 wt %.
[0070] Amo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com