1-styryl isoquinoline derivative as well as preparation and application thereof
A styryl, methyl isoquinoline technology, applied in the field of medicine, can solve the problems of insignificant early symptoms of gastric cancer, low survival rate of gastric cancer, etc., and achieve inhibition of gastric cancer cell migration and invasion, significant anti-gastric cancer activity, and good anti-gastric cancer. active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
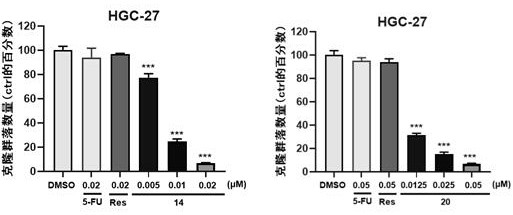
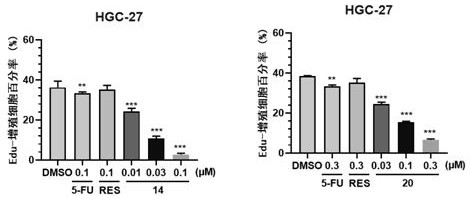
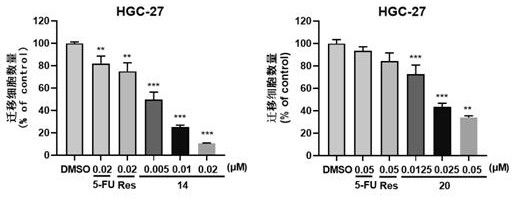
Image
Examples
Embodiment 1
[0034] (E)-1-(2-fluorostyryl)isoquinoline (compound 1)
[0035] The structural formula of compound 1 is:
[0036] Put 1-methylisoquinoline (1.0 mol), o-fluorobenzaldehyde (1.1 mol), and p-toluenesulfonamide (1.1 mol) in a 50 mL round-bottomed flask, and replace it with argon, add 10 mL of toluene, and Heat to reflux at 120°C and react for 8 hours. After the completion of the reaction monitored by TLC, the solvent was removed by rotary evaporation, and the compound (E)-1-(2-fluorostyryl)isoquinoline (compound 1) was obtained by column chromatography separation with a yield of 67%. 1 H NMR (400 MHz, DMSO- d 6 ) δ 8.63 (d, J = 8.3Hz, 1H), 8.55 (d, J = 5.5 Hz, 1H), 8.32 (d, J = 15.6 Hz, 1H), 8.21 – 8.11 (m,2H), 7.93 (d, J = 7.8 Hz, 1H), 7.78 – 7.64 (m, 3H), 7.38 (ddd, J = 12.6, 5.7,2.7 Hz, 1H), 7.31 – 7.21 (m, 2H). 13 C NMR (101 MHz, DMSO- d 6 ) δ 160.27 (d, J =248.9 Hz), 153.24, 142.25, 136.22, 130.30 (d, J = 8.5 Hz), 130.09, 128.08 (d, J = 3.0 Hz), 127.49...
Embodiment 2
[0038](E)-1-(2-chlorostyryl)isoquinoline (Compound 2)
[0039] The structural formula of compound 2 is:
[0040] The o-fluorobenzaldehyde in the operating steps of Example 1 was replaced by o-chlorobenzaldehyde, and all the other operating steps were the same as in Example 1, with a yield of 71%. 1 H NMR (400 MHz, Chloroform- d ) δ 8.57 (d, J = 5.6 Hz, 1H), 8.33(d, J = 15.5 Hz, 1H), 8.29 (d, J = 8.4 Hz, 1H), 7.93 (d, J = 15.6 Hz, 1H),7.84 – 7.76 (m, 2H), 7.68 – 7.61 (m, 1H), 7.60 – 7.50 (m, 2H), 7.41 (m, 1H),7.28 (td, J = 7.6, 1.4 Hz, 1H), 7.25 – 7.18 (m, 1H). 13 C NMR (101 MHz, DMSO- d 6 ) δ 154.11, 142.50, 136.57, 135.14, 134.28, 131.94, 129.92, 129.79, 129.29, 127.23, 127.19, 127.18, 126.83, 126.68, 120.16.
Embodiment 3
[0042] (E)-1-(2-bromostyryl)isoquinoline (compound 3)
[0043] The structural formula of compound 3 is:
[0044] The o-fluorobenzaldehyde in the operating steps of Example 1 was replaced by o-bromobenzaldehyde, and all the other operating steps were the same as in Example 1, and the productive rate was 83%. 1 H NMR (400 MHz, Chloroform- d ) δ 8.59 (d, J = 5.6 Hz, 1H), 8.37 –8.24 (m, 2H), 7.90 (d, J = 15.5 Hz, 1H), 7.81 (m, J = 7.9, 1.6 Hz, 2H), 7.70– 7.59 (m, 2H), 7.57 (d, J = 5.8 Hz, 1H), 7.36 (t, J = 7.5 Hz, 1H), 7.17 (m, J = 7.7, 1.6 Hz, 1H). 13 C NMR (101 MHz, Chloroform- d ) δ 154.16, 142.61, 137.03, 136.65, 134.63, 133.24, 129.87, 129.59, 127.55, 127.44, 127.30, 127.26, 126.75, 126.10, 124.808, 2.124
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com