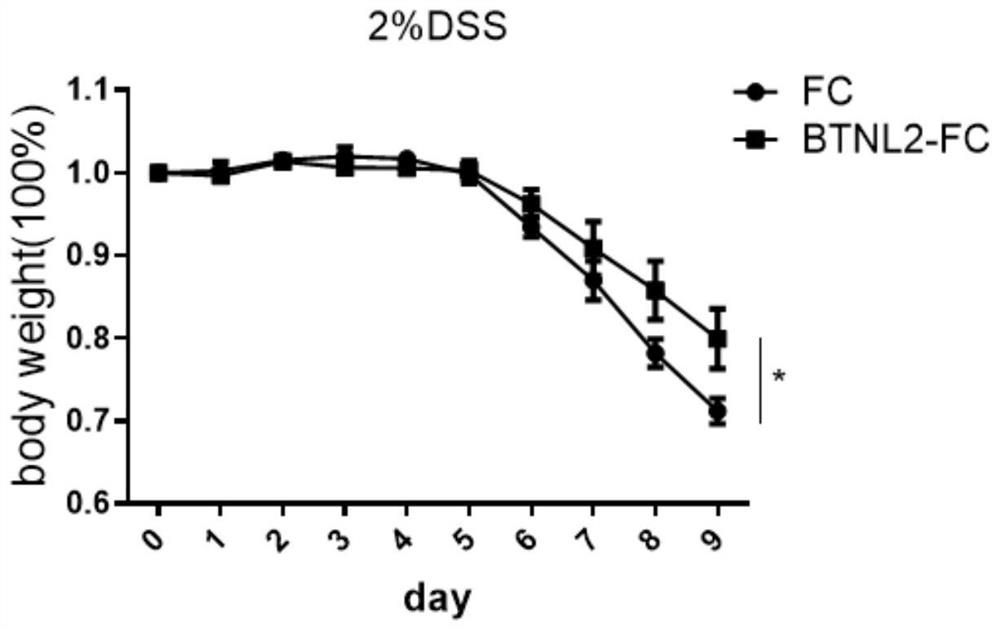
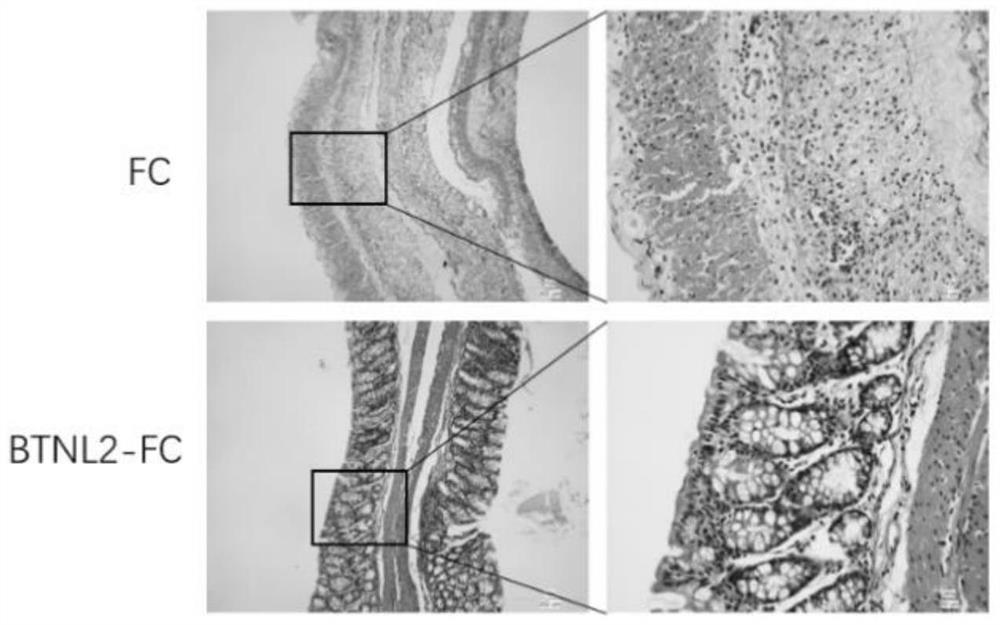
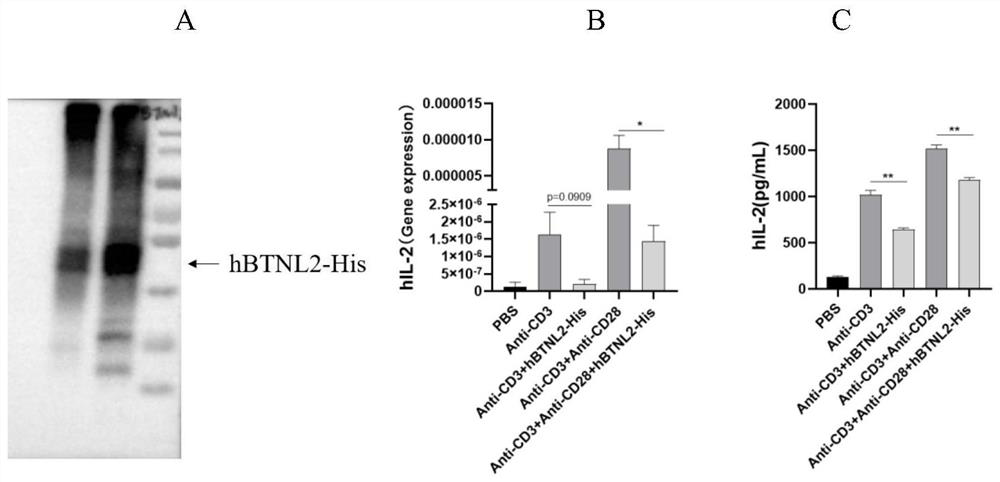
BTNL2 recombinant protein, preparation method thereof and application of BTNL2 recombinant protein in preparation of medicine for treating inflammatory bowel disease
A recombinant protein and drug technology, applied in the field of biomedicine, can solve the problems of persistent disease, lack of treatment methods, and increased risk of colorectal cancer, and achieve the effects of reducing enteritis, reducing mortality, and reducing weight loss in mice
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1, the preparation of recombinant protein BTNL2-Fc
[0043] 1. Construction of expression vector
[0044]The full cDNA sequence of mouse BTNL2-Fc was obtained by synthesis, wherein the nucleotide sequence of the extracellular region of mouse BTNL2 (27-452) is shown in SEQ ID NO.6, and the nucleotide sequence of the recombinant protein BTNL2-Fc is shown in SEQ ID NO. Shown in .1; Utilize EcoRI and NheI to digest the pFUSE-hIgG2-Fc vector (purchased from Invivogen), excise the 720bp fragment, and reclaim the 3424bp vector fragment; synthesize the BTNL2-Fc containing EcoRI and NheI restriction sites The entire cDNA sequence was digested with EcoRI and NheI and cloned into the recovered vector fragment by T4 DNA ligase, and the formed vector was named pINFUSE-mBTNL2-hIgG2-Fc.
[0045] 2. Transfection, expression and purification
[0046] The constructed expression vector was transfected into 293F cells (1L cultured cells, 1×10 6 / ml), at 37°C, 5% CO 2 , 130rp...
Embodiment 2
[0048] Embodiment 2, the preparation of recombinant protein BTNL2-his
[0049] 1. Construction of expression vector
[0050] Obtain the full cDNA sequence of human BTNL2 by synthesis, wherein the amino acid encoded by the extracellular region is the 24th lysine to the 453rd serine of human BTNL2, the nucleotide sequence of the human BTNL2 extracellular region nucleic acid sequence (24-453) As shown in SEQ ID NO.5;
[0051] The PET28a vector was digested with BamHI and XhoI, and the complete cDNA sequence of human BTNL2 was synthesized as a template, and primers F-5'-CGGGATCCATGAAGCAGTCAGAAGACTTTAG-3'(SEQ ID NO.3), R-5'-CCGCTCGAGT CTAATGGTGATGGTGATGATGTGAGAGAGAAAAAGTTG-3'( SEQ ID NO.4) was performed to obtain the full cDNA sequence of human BTNL2-His by PCR, which was digested with BamHI and XhoI and cloned into the vector fragment by T4DNA ligase, and the formed vector was named PET28a-hBTNL2-His. The nucleotide sequence of the recombinant protein BTNL2-his is shown in SEQ I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com