Improved preparation method of Rhodomyrtone
A compound and reaction technology, applied in the field of improved preparation of Rhodomyrtone, can solve the problems of low total yield, a large number of explosive and toxic solvents, difficult application, etc., and achieve the effects of cheap solvent, easy synthesis process, and high synthesis yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0048] In order to further illustrate the technical effect of the present invention, the present invention will be described in detail below through embodiments. The examples provided are merely illustrative of the methods of the present invention, and are not intended to limit the disclosure of the present invention in any way.
[0049] Unless otherwise specified, the reagents, methods and equipment used in the present invention are conventional reagents, methods and equipment in the art. The compound represented by formula I below is referred to as compound I, the compound represented by formula II is referred to as compound II, and so on.
[0050] In the following examples, compounds II to IV are used as intermediate products, and the purity is greater than 90% after detection, close to 100%, so the amount of the substance is calculated according to the purity of 100%.
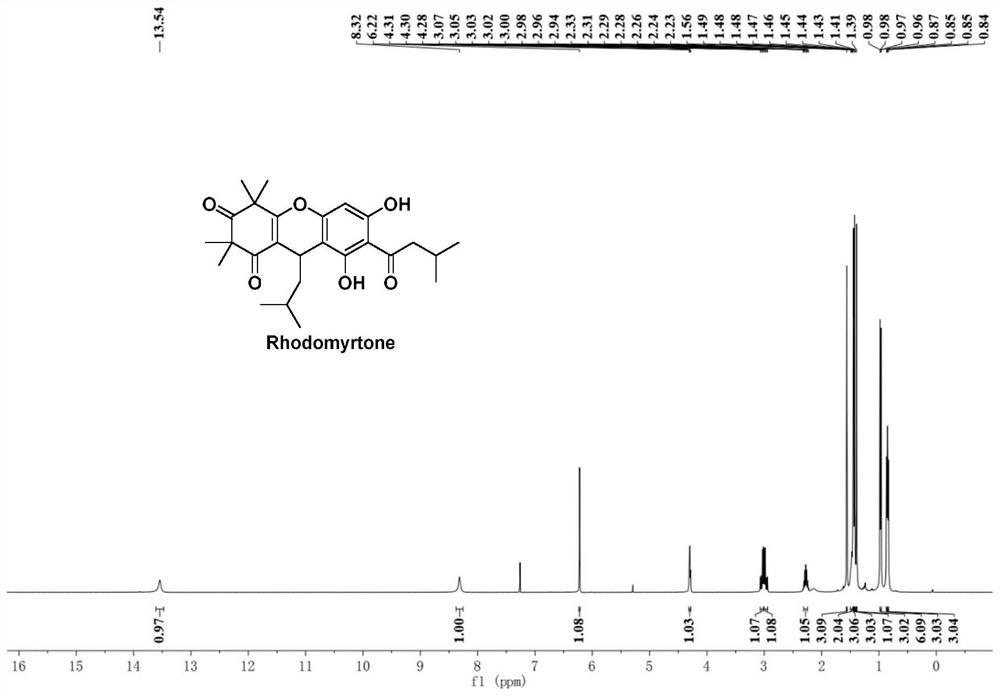
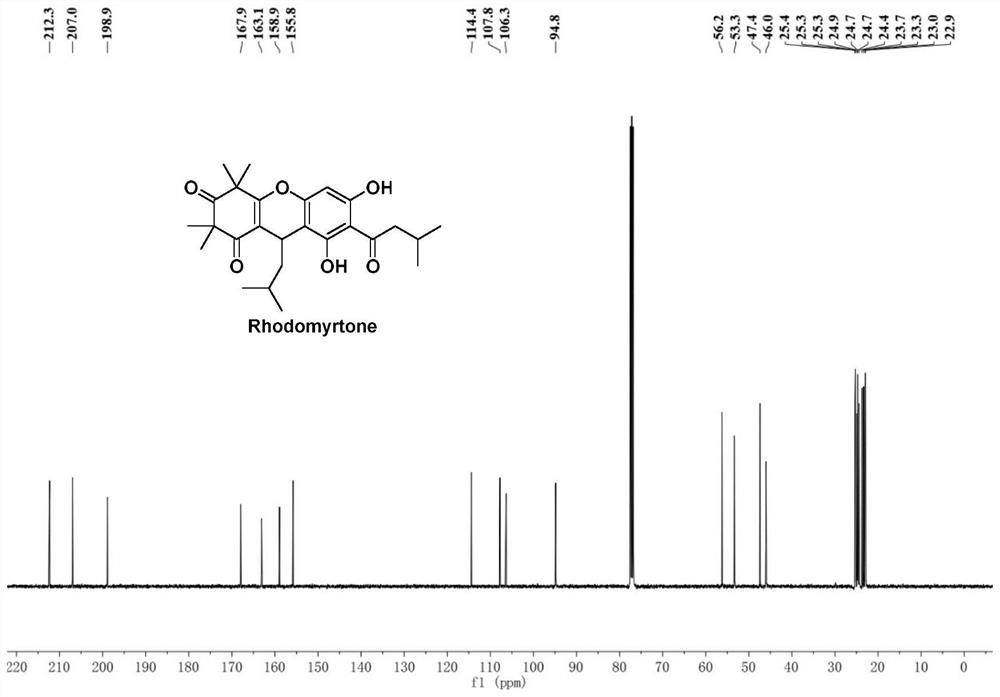
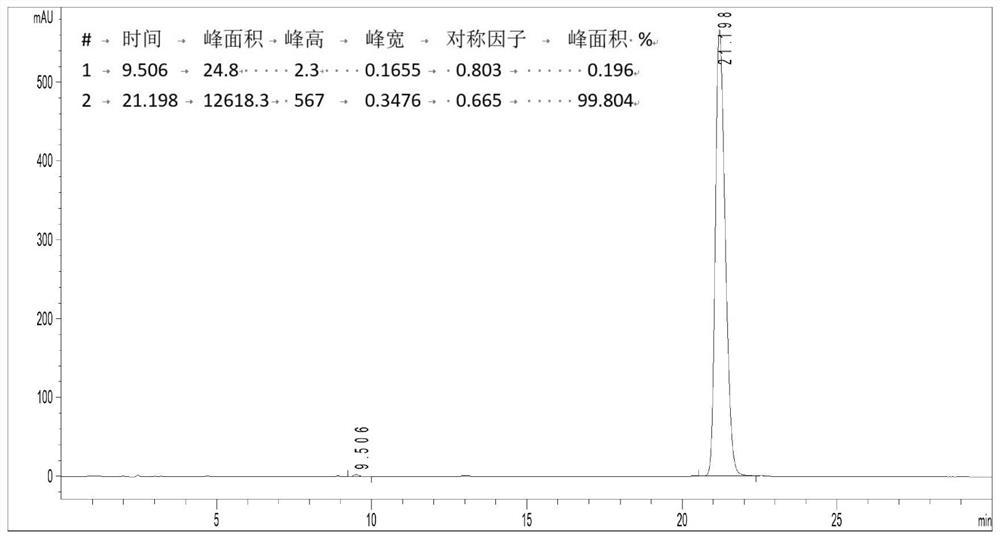
[0051] Example Preparation of Rhodomyrtone
[0052] The compound shown in the formula I of the embodim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com