Antibodies against claudin 18.2 for cancer diagnosis
一种抗体、抗原的技术,应用在用于癌症诊断的针对密蛋白18.2的抗体领域,能够解决表达局限等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0158] Sample preparation is critical for maintaining cell morphology, tissue structure, and antigenicity of target epitopes. This requires proper tissue collection, fixation and sectioning. Usually paraformaldehyde is used for fixation. Depending on the purpose and thickness of the experimental sample, thin (about 4 to 40 μm) sections are cut from the tissue of interest, or can be used in its entirety if the tissue is not very thick and penetrable. Sectioning is typically accomplished by using a microtome, and the sections are mounted on slides.
[0159] Samples may require additional steps to make epitopes available for antibody binding, including deparaffinization and antigen retrieval. Typically, detergents (eg, Triton X-100) are used in immunohistochemistry to reduce surface tension so that less reagents can be used to achieve better and more uniform coverage of the sample.
[0160] A straightforward method for immunohistochemical staining is to use a labeled antibody ...
Embodiment 1
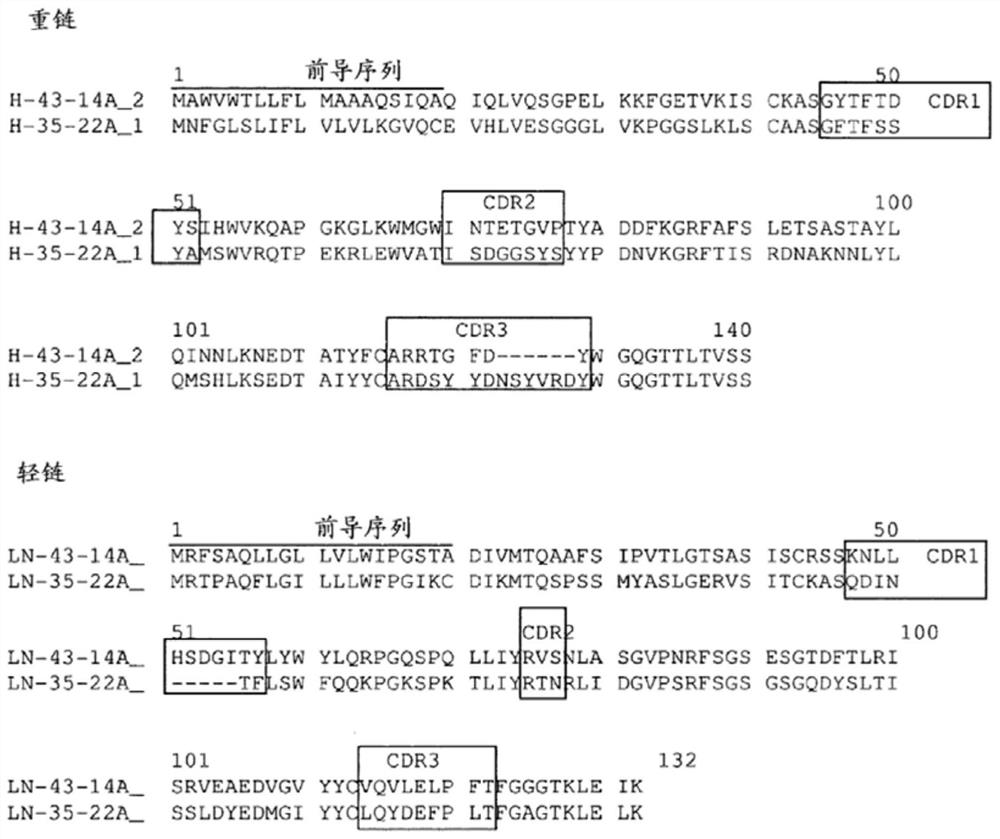
[0255] Example 1: Production of Monoclonal Antibodies
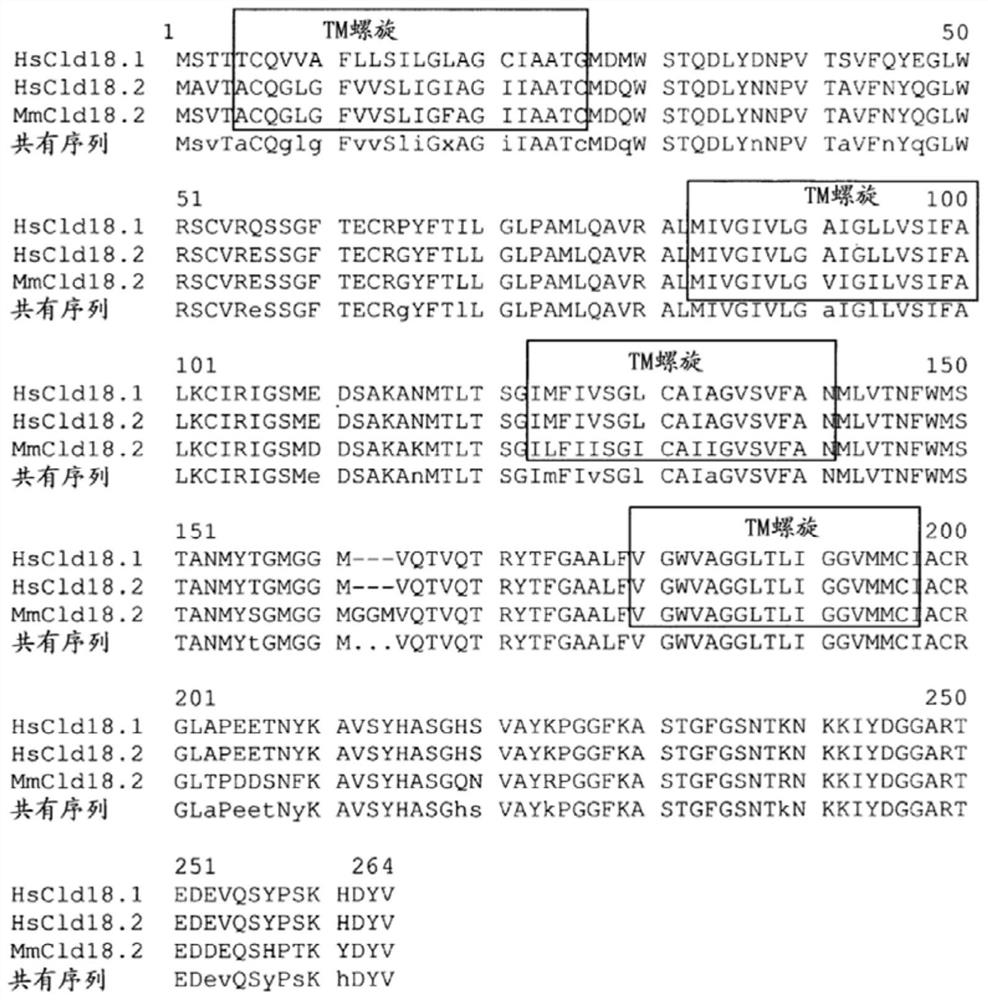
[0256] The objective of this project is to generate mouse CLDN-18-specific monoclonal antibodies capable of detecting CLDN18.2-expressing tumor cells in gastric CA, esophageal CA, pancreatic CA, and lung CA FFPE tissues.
[0257] To generate highly specific, high affinity diagnostic CLDN18.2 antibodies, it is necessary to initiate immunization regimens with a wide variety of different immunogens and adjuvants. During this project, approximately 100 mice (C57B1 / 6 and Balb / c) were vaccinated using various immunization strategies to elicit α-CLDN18 immune responses.
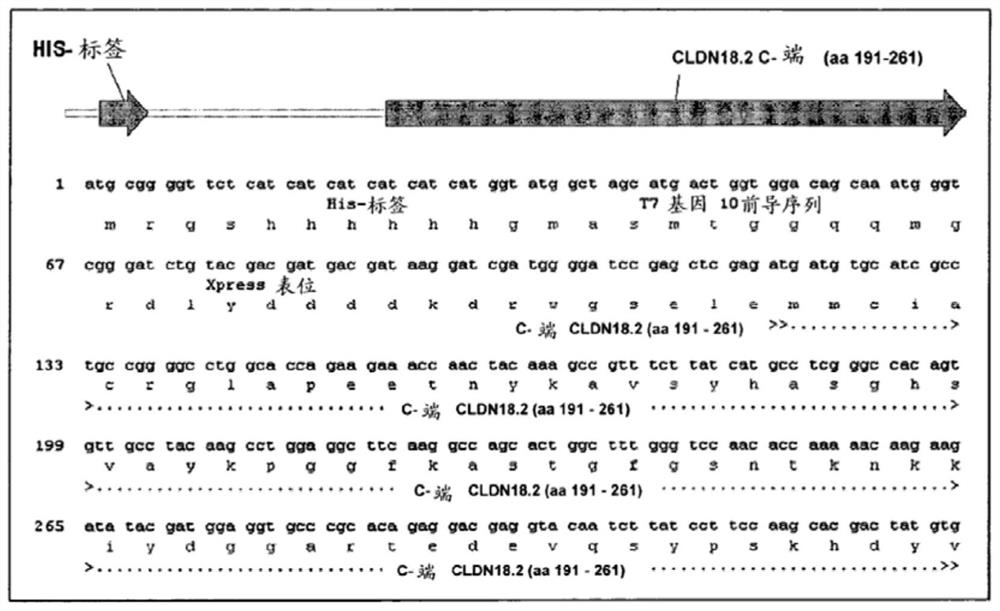
[0258] To elicit the mouse immune system and overcome immune tolerance, we employed virus-like particles (VLPs), peptide conjugates, and recombinant proteins encoding different parts of human CLDN18.2 expressed with different expression partners ( tag) recombinant fusion protein.
[0259] In 13 different immunization strategies, HIS-tagged C-terminal recombin...
Embodiment 2
[0264] Example 2: Western blot screening of monoclonal hybridoma supernatants
[0265] To answer the question of whether ELISA-positive antibodies in the supernatant could bind to recombinant claudin 18 or protein lysates from stably transfected HEK293 cells expressing claudin 18, Western blot analysis was performed. Antibodies capable of binding specifically to claudin 18 in Western blot analysis were amplified. Cells were frozen and antibodies were purified by MABselect (FPLC). Antibodies selected by Western blot screening were purified and evaluated by immunohistochemistry for their ability to bind their antigens in formalin-fixed paraffin-embedded tissue (FFPE).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com