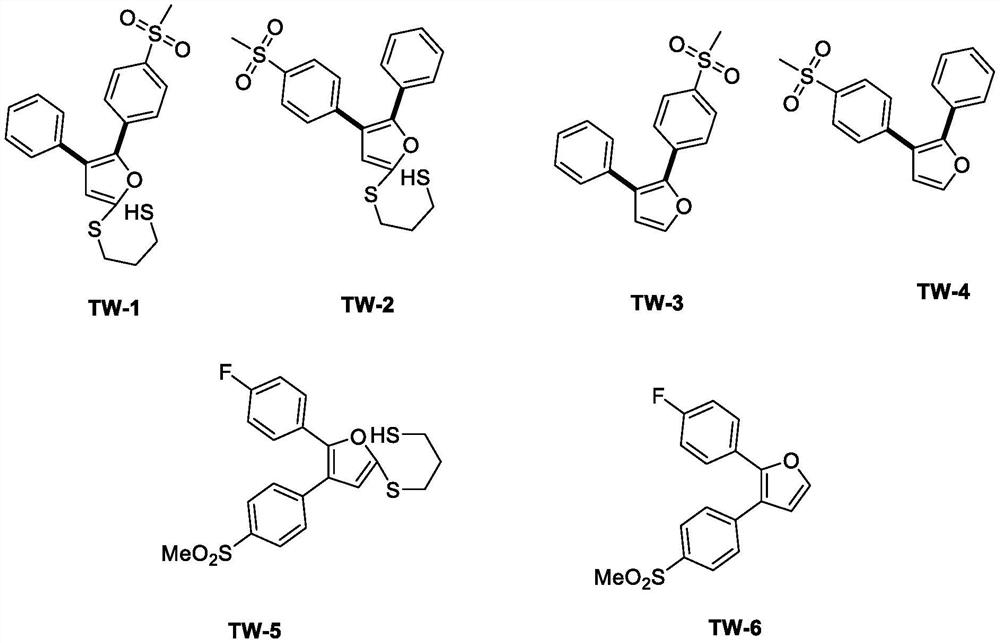
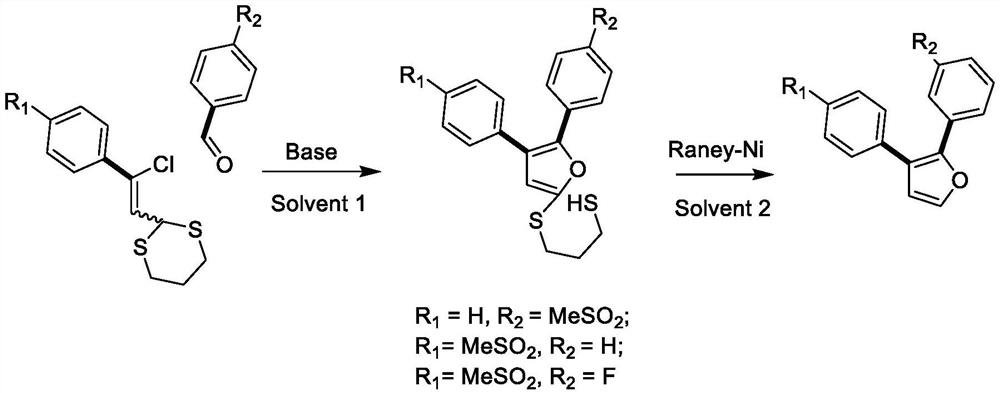
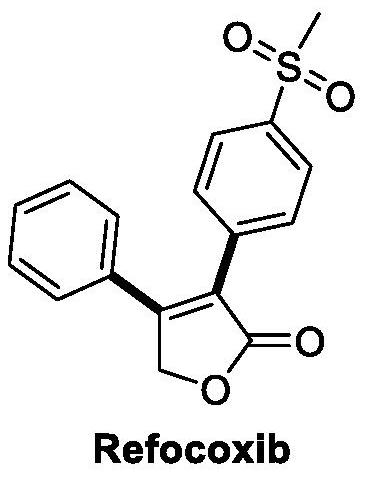
Design and preparation method of novel rofecoxib derivative
A technology of rofecoxib and derivatives, which is applied in the field of preparation of rofecoxib furan analogs, and can solve problems such as increasing the risk of heart disease or stroke
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 2: The reaction steps of the method for preparing novel rofecoxib derivative TW-2:
[0019] Add 4 mL of solvent MeCN, compound 4-thiamphenicol phenyl-β-chloro-ethylene-1,3-dithiane (167 mg) and 4-thiamphenicol benzaldehyde (53 mg) into the reaction flask, and add KOH ( 34 mg), reacted at room temperature for 10 minutes, added dilute acid to adjust the pH to neutral, separated and purified to obtain the target compound TW-2 (151 mg, 75%).
[0020] The structure of embodiment 2 gained product TW-2, nuclear magnetic data are as follows:
[0021] 1H NMR (400MHz, Chloroform-d) δ7.90 (d, J=8.5Hz, 2H), 7.60-7.55(m, 2H), 7.51-7.46(m, 2H), 7.41-7.38(m, 2H), 7.28(dd, J=8.6, 1.9Hz, 1H), 6.68(s, 1H), 3.09(s, 3H), 3.00(t, J=7.0Hz, 2H), 2.75–2.68(m, 2H), 1.99 (q,J=7.0Hz,2H),1.37(t,J=8.1Hz,1H).
[0022] Embodiment 3: The reaction steps of the method for preparing novel rofecoxib derivative TW-3:
Embodiment 2
[0023] TW-1 (81 mg) was dissolved in 10 mL of methanol and placed in a reaction flask, and Raney nickel (290 mg) was added to react for 24 h for reduction to obtain the target compound TW-3 (53 mg, 89%).
[0024] The structure of embodiment 3 gained product TW-3, nuclear magnetic data are as follows:
[0025] 1H NMR (400MHz, Chloroform-d) δ7.88–7.76(m,2H),7.75–7.66(m,2H),7.57(d,J=1.8Hz,1H),7.47–7.33(m,5H), 6.65– 6.52(m,1H),3.05(s,4H).13C NMR(101MHz,Chloroform-d)δ146.34, 142.91,138.54,136.19,133.56,128.96,128.81,128.72,128.62,127.92, 1226.20, ,126.09,125.62,114.82,44.48.
[0026] Embodiment 4: The reaction steps of the method for preparing novel rofecoxib derivative TW-4:
[0027] TW-2 (83 mg) was dissolved in 10 mL ethanol and placed in a reaction flask, and Raney nickel (297 mg) was added to react for 24 h for reduction to obtain the target compound TW-4 (54 mg, 89%).
Embodiment 3
[0029] 1H NMR (400MHz, Chloroform-d) δ7.97–7.80(m,2H),7.69–7.43(m,4H),7.38–7.20(m,3H),6.58(d,J=2.1Hz,1H), 3.08(s, 3H).13C NMR(101MHz,CDCl3)δ146.34,142.91,138.54,136.19,133.56, 128.96,128.81,128.72,128.62,128.09,127.92,127.56,126.20,126.09, 125.62,114.82,77.36,77.04, 76.73, 44.48.
[0030] Embodiment 5: The reaction steps of the method for preparing novel rofecoxib derivative TW-6:
[0031] Add 4mL DMSO, compound 4-thiamphenylphenyl-β-chloro-ethylene-1,3-dithiane (167mg) and 4-fluorobenzaldehyde (62mg) into the reaction flask, add tBuOK (67mg) under stirring, React at room temperature for 10 minutes, add dilute hydrochloric acid to adjust the pH to neutral, separate and purify to obtain TW-5 compound, spin to dry the solvent, add methanol, add Raney nickel (299mg), react for 24h, and obtain TW-6 (41mg, 65% )
[0032] The structure of embodiment 5 gained product TW-6, nuclear magnetic data are as follows:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com