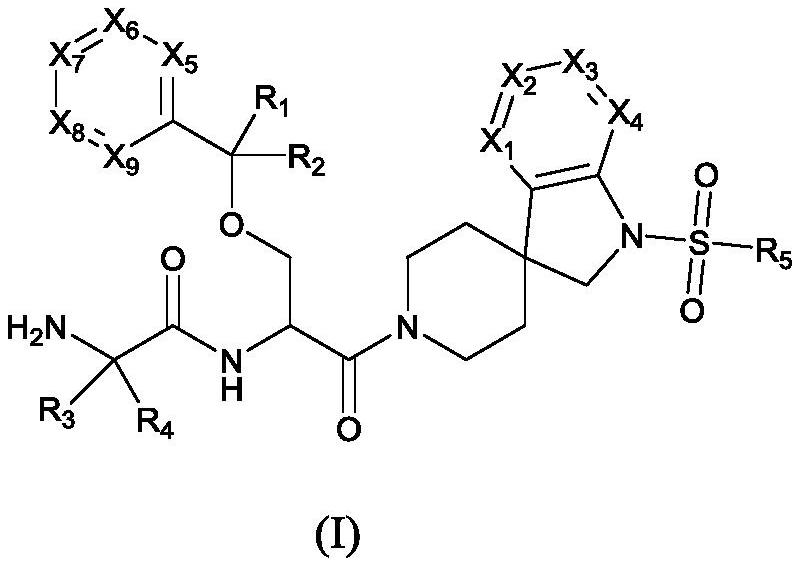
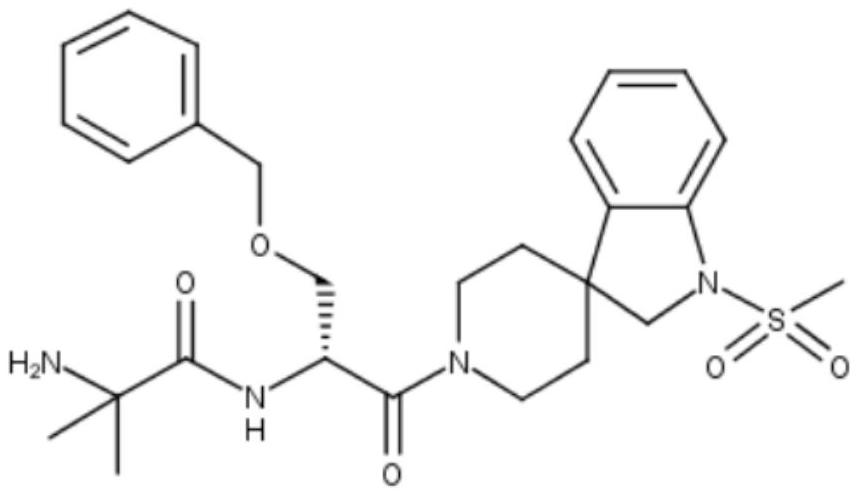
Growth hormone secretagogue derivative and preparation method thereof
A technology of drugs and compounds, applied in the field of medicine, can solve problems such as short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
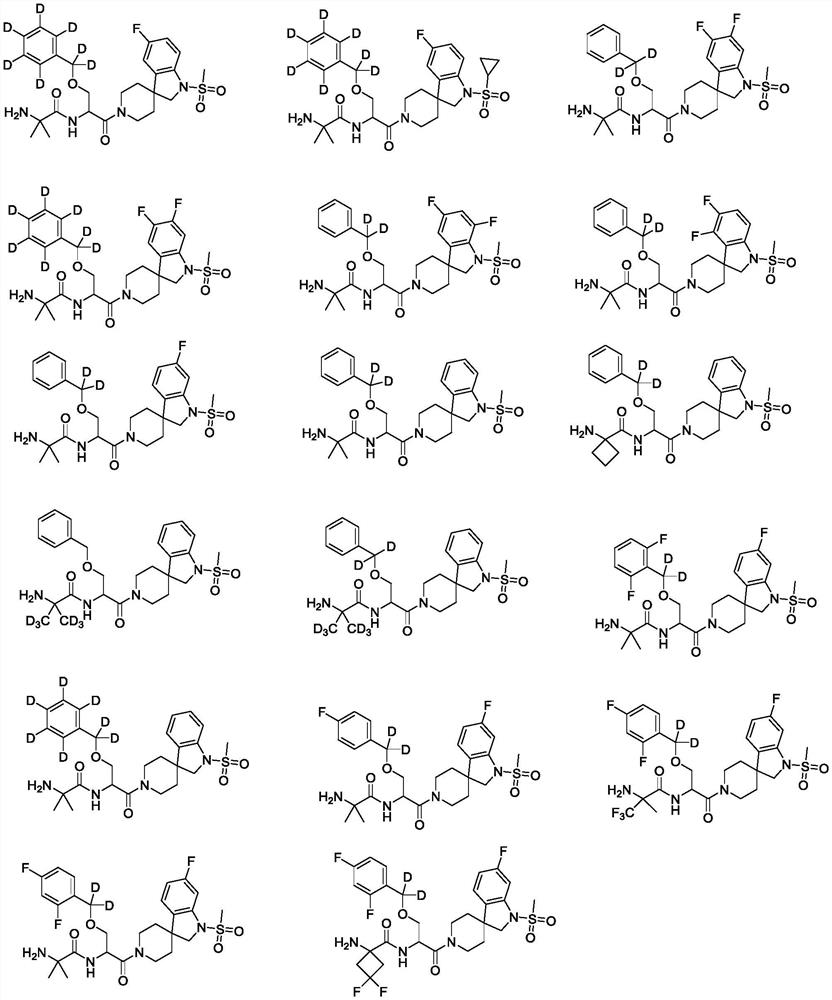
[0116] (R)-2-Amino-N-(1-(5-fluoro-1-(methylsulfonyl)spiro[indoline-3,4'-piperidin]-1'-yl)-1-carbonyl -3-((Phenyl-d5)methoxy-d2)propan-2-yl)-2-methylpropanamide (1)
[0117]
[0118] The synthesis of intermediate BB-1 is as follows:
[0119]
[0120] first step:
[0121] Lithium bromide (754 mg, 8.68 mmol) was suspended in dry acetonitrile (10 mL), trimethylsilyl chloride (1.18 g, 10.85 mmol) was added, and finally benzyl alcohol-d7 (500 mg, 4.34 mmol) was added, and the reaction was heated under reflux. After monitoring the completion of the reaction by TLC, it was cooled to room temperature, water (10 mL) was added, extracted with ethyl acetate, the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to crude product BB (700 mg, yield 91%). ).
[0122] Step 2:
[0123] Sodium hydride (60%, 354 mg, 14.74 mmol) was suspended in dry DMF (6 mL), cooled to 0°C, and a solution of tert-...
Embodiment 2
[0150] (R)-2-Amino-N-(1-(1-(cyclopropylsulfonyl)-5-fluorospiro[indoline-3,4'-piperidin]-1'-yl)-1 -Carbonyl-3-((phenyl-d5)methoxy-d2)propan-2-yl)-2-methylpropionamidecarboxylate (2)
[0151]
[0152] Step 1: Benzyl 1-(Cyclopropylsulfonyl)-5-fluorospiro[indoline-3,4'-piperidine]-1'-carboxylate (2a)
[0153] 1a (1.50 g, 4.41 mmol) was dissolved in dichloromethane (10 mL), triethylamine (1.53 mL, 11.0 mmol) and 4-dimethylaminopyridine (53.8 mg, 441.0 umol) were added, and cooled to 0°C. Cyclopropanesulfonyl chloride (718 μL, 7.05 mmol) was added, and the temperature was raised to 40° C. to react. After the completion of the reaction monitored by TLC, water was added to quench the reaction, extracted with ethyl acetate, the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to a crude product, which was subjected to flash column chromatography to obtain 2a (1.5 g , the yield is 77%).
[0...
Embodiment 3
[0165] (R)-2-Amino-N-(1-(5,6-difluoro-1-(methylsulfonyl)spiro[indoline-3,4'-piperidin]-1'-yl)- 1-Carbonyl-3-((phenyl-d5)methoxy-d2)propan-2-yl)-2-methylpropionamidecarboxylate (3)
[0166]
[0167] According to the synthetic method of Example 1, the starting material 4-fluorophenylhydrazine was replaced with 3,4-difluorophenylhydrazine, and the title compound 3 was obtained as the final product by formic acid system.
[0168] MS(ESI): m / z=572.1[M+H] +
[0169] 1 H NMR (400MHz, CD 3 OD)δ8.54(s,1H),7.36-6.58(m,2H),5.23-5.06(m,1H),4.60-4.45(m,1H),4.20-4.02(m,1H),3.96(s) ,2H),3.80–3.68(m,2H),3.28–3.13(m,1H),3.07–2.97(m,3H),2.92–2.75(m,1H),2.02–1.58(m,4H),1.56 –1.45(m,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com