IL-10/FC fusion proteins useful as enhancers for immunotherapy
A technology of fusion protein and immunotherapy, applied in immunoglobulin, peptide/protein components, animal/human protein, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Example 1: Preparation of Fc fusion protein IL-10 / Fc of the present invention
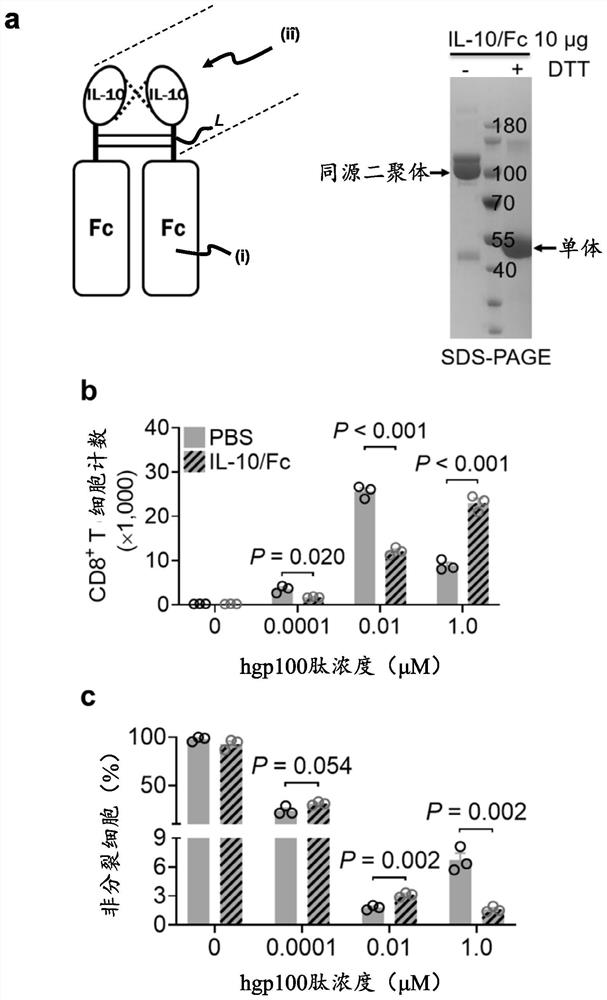
[0122] For recombinant human IL-10 and IgG1 Fc fusion protein (IL-10 / Fc of SEQ ID NO: 4) ( figure 1 a) processed to cross-react with mouse IL-10 receptor or human IL-10 receptor (IL-10R) via the IL-10 domain of the IL-10 / Fc fusion protein (Qiao et al., 2019, Cancer Cell 35, 901–915.e4). Therefore, the IL-10 / Fc fusion protein can act on both mouse and human CD8+ T cells.
[0123] IL-10 / Fc fusion proteins were obtained by using a gene carrying an IL-10 / Fc fusion gene (as previously described in Guo et al., 2012, or in Zheng et al., 1997, J. Immunol., 158, 4507–4513 or in Commercial mammalian expression vectors such as pcDNA3.1 or pSectag2A described in Steele et al., 1995, J. Immunol., 154, 5590–5600) were expressed from HEK293 free style cells and then harvested by centrifugation after 7 days in culture containing Culture supernatant of IL-10 / Fc.
[0124] The IL-10 / Fc fusion protein was f...
Embodiment 2
[0125] Example 2: The role of fusion protein IL-10 / Fc of the present invention in IL-10-mediated metabolic reprogramming
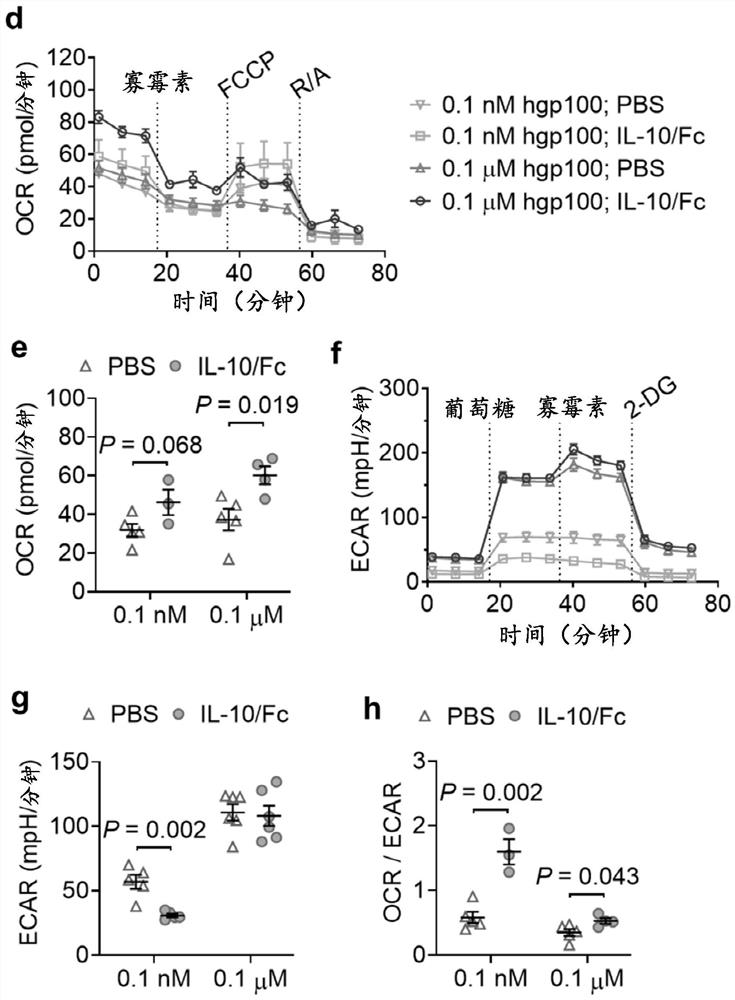
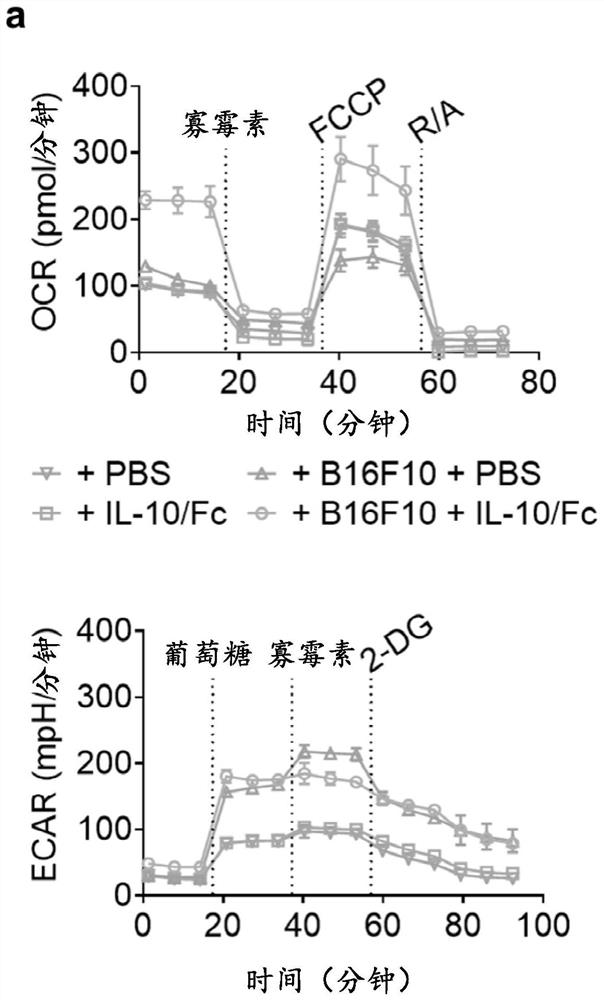
[0126]In a co-culture system of B16F10 mouse melanoma cells and activated Pmel CD8+ T cells recognizing gp100 cognate antigens, the basal and maximal oxygen consumption rate (OCR) of CD8+ T cells were determined using the fusion protein IL-10 / Fc of the present invention. Both increased after treatment, while the extracellular acidification rate (ECAR) remained unchanged ( figure 2 a-c). The ratio of OCR to EACR was significantly increased in CD8+ T cells treated with IL-10 / Fc ( figure 2 d), which indicates that IL-10 signaling actively promotes T cell oxidative phosphorylation (OXPHOS). Consequently, T cell counts and cytotoxicity are greatly enhanced ( figure 2 e and figure 2 f). Importantly, this metabolic reprogramming effect was not observed in CD8+ T cells without antigen stimulation ( figure 2 d), which suggests that IL-10-mediated metabol...
Embodiment 3
[0129] Example 3: In vivo anti-tumor effect of the fusion protein of the present invention
[0130] Enhancement of OXPHOS or inhibition of glycolytic metabolism in CD8+ T cells by various agents promoted CD8+ T cell proliferation, memory development and antitumor function in the TME (Zhang et al., 2017, Cancer Cell 32, 377–391.e9; Chowdhury et al., 2018, Cancer Immunol. Res. 6, 1375-1387; Sukumar et al., 2013, 123, 4479-4488). Based on the observed metabolic regulation effect of the Fc fusion protein of the present invention on CD8+ T cells, in order to improve the efficacy of T cell adoptive immunotherapy on solid tumors, whether IL-10 / Fc can achieve in vivo metabolic intervention of CD8+ T cells Were studied.
[0131] To overcome T cell depletion in the TME, the in vivo antitumor effects of IL-10 / Fc together with TCR transgenic CD8+ T cells (Pmel CD8+ T cells or OTI CD8+ T cells) or ACT of HER2 CAR-T cells were shown in several studies. tumor models such as B16F10 (low imm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com