Anti-aggregation agent, and pharmaceutical composition and medical device using same
A technology of aggregating agent and composition, which can be used in drug combinations, pharmaceutical formulations, medical preparations of inactive ingredients, etc., and can solve problems such as difficulty in aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] (Example 1: Test suspension containing aripiprazole)
Embodiment 1-1-1
[0076] (Example 1-1-1: Preparation and evaluation of test suspension using polyoxyethylene cetyl ether)
[0077] Aripiprazole hydrate, sodium carboxymethyl cellulose, mannitol, and sodium dihydrogen phosphate monohydrate, which are poorly water-soluble drugs, were added to purified water to achieve the following concentrations to prepare an aripiprazole suspension.
[0078] ・Aripiprazole hydrate: 30% by mass (as anhydrous);
[0079] ・Sodium carboxymethylcellulose: about 1.248% by mass;
[0080] ・Mannitol: about 6.24% by mass;
[0081] ・Sodium dihydrogen phosphate monohydrate: about 0.111% by mass.
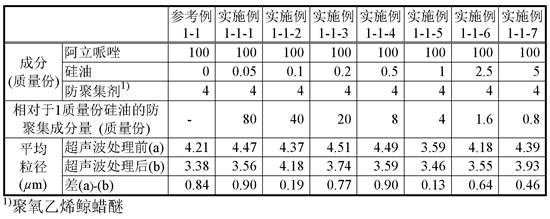
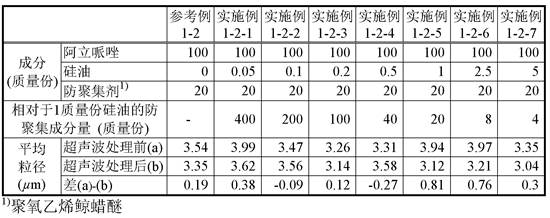
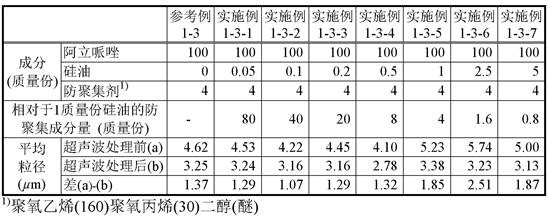
[0082] To 1 g of the aripiprazole suspension, only an amount equivalent to 4 parts by mass of polyoxyethylene cetyl ether was added as an anti-aggregation agent with respect to 100 parts by mass of aripiprazole contained in the suspension, and the Stir. To the suspension was added 1% silicone oil emulsion (Dow Corning (registered trademark) 365, 35% dimethicone NF Elusion; here...
Embodiment 1-1-2~1-1-7
[0083] (Examples 1-1-2 to 1-1-7: Preparation and evaluation of test suspensions using polyoxyethylene cetyl ether)
[0084] To the test suspension (E1-1-1) (suspension before ultrasonic treatment) obtained in Example 1-1-1, 1% of silicone oil emulsion was added, respectively, so that the silicone oil contained in the suspension was adjusted to 100% by mass. Part of aripiprazole was in the amount shown in Table 1, and the test suspensions (E1-1-2) to (E1-1-7) were prepared to obtain the test suspensions (E1-1-2) to (E1 -1-7). The particle sizes of particles contained in the test suspensions before (a) and after (b) ultrasonication were measured for each test suspension. The results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com