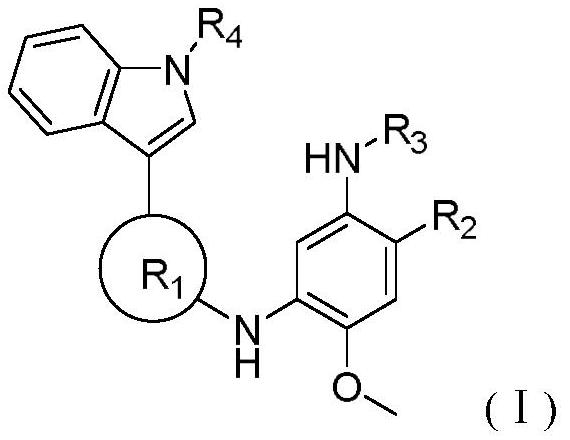
4-methoxyphenyl-1, 3-diamine derivative containing 1-methyl-1H-indole structure and application of 4-methoxyphenyl-1, 3-diamine derivative
A technology of methoxyphenyl and diamine derivatives, which can be used in medical preparations containing active ingredients, drug combinations, organic chemistry, etc., and can solve problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
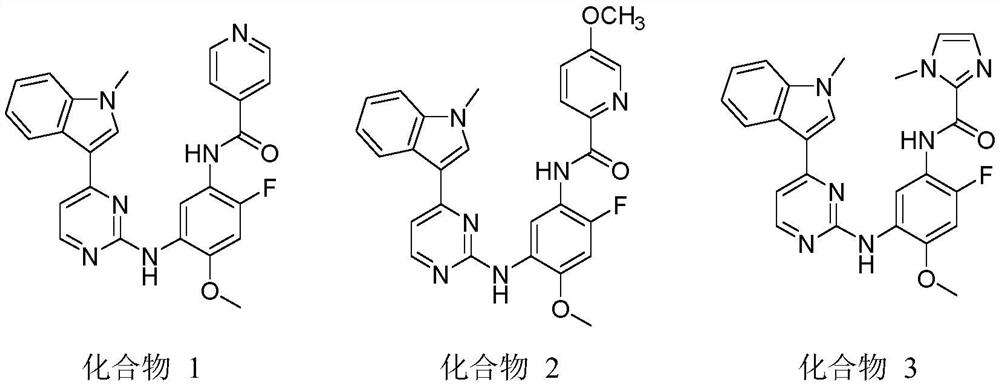
[0034] Preparation of Example 1 Compound 1
[0035]
[0036] Step 1: Preparation of Intermediate c
[0037] 2,4-Dichloropyrimidine (5.00 g, 1 eq), anhydrous AlCl 3 (5.40g, 1.2eq) was added to the reaction flask, 1,4-dioxane (30mL) was added to dissolve, and the mixture was stirred in an oil bath at 60°C under nitrogen protection until it was completely dissolved. After complete dissolution, N-methyl indole (5.30 g, 1.2 eq) was added by injection and stirred for about 15 min. After the reaction was detected by TLC, the reaction solution was added to 10 times the amount of ice water, and an orange-yellow solid was precipitated, which was washed with suction and dried in a vacuum oven at 50°C. The dried solid was separated and purified by silica gel column chromatography to obtain 6.69 g of off-white solid, namely intermediate c, with a yield of 81.8%. This solid can be used directly in the next reaction.
[0038] Step 2: Preparation of Intermediate d
[0039] Take the in...
Embodiment 10
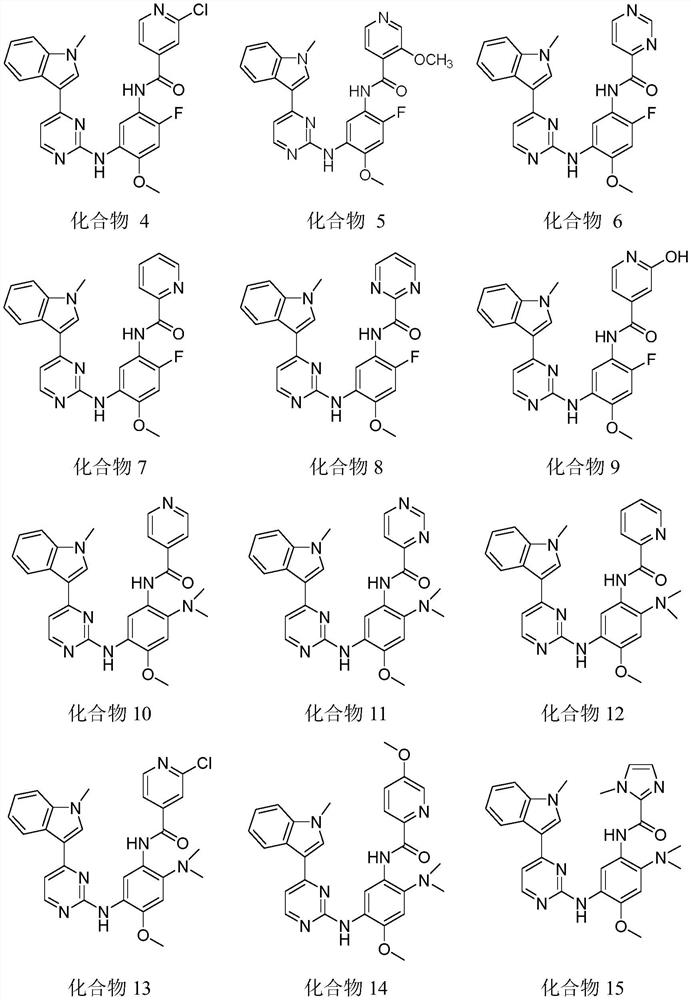
[0049] Example 10 Preparation of Compound 10
[0050]
[0051] Wherein, intermediate c and intermediate d can be prepared by referring to the method in Example 1.
[0052] Step 1: Synthesis of Intermediate f
[0053] Get intermediate d (3.50g, 1eq), K in above-mentioned embodiment 1 2 CO 3 The solid (2.58g, 2eq) was placed in a 50mL round-bottom flask, DMF (20.0mL) was added to dissolve it, transferred to an oil bath at 90°C, and then dimethylamine hydrate (0.633g, 1.5eq) was added dropwise with stirring. The reaction was continued for about 6 h, and the reaction was completed after TLC dot plate detection. The reaction solution was cooled to room temperature, extracted three times with DCM, the organic phases were combined, washed three times with saturated aqueous NaCl solution, and anhydrous Na 2 SO 4 Dewatering and drying. Spin-dried under reduced pressure to obtain 3.304 g of a red solid product, namely intermediate f, with a yield of 88.8%. This solid can be us...
Embodiment 20
[0063] Example 20 Preparation of Compound 20
[0064]
[0065] Among them, intermediate c and intermediate d can be synthesized according to the method in Example 1.
[0066] Step 1: Synthesis of Intermediate h
[0067] Take the intermediate d (3.00g, 1eq) in the above example 1 and place it in a 50mL round bottom flask, add DMF (30mL) to dissolve it, add K 2 CO 3 The solid (2.10g, 2eq) was transferred to an oil bath at 90°C, and N,N,N-trimethylethylenediamine liquid (1.16g, 1.5eq) was added dropwise to start the reaction. After 6h, the reaction was complete by TLC dot plate detection. The reaction solution was cooled to room temperature, extracted three times with DCM, the organic phases were combined, washed three times with saturated aqueous NaCl solution, and anhydrous Na 2 SO 4 Dewatering and drying. Spin-dried under reduced pressure to obtain 3.23 g of a red solid product, namely intermediate h, with a yield of 90.2%. This solid can be used directly in the next...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com