Preparation and anti-tumor effect of RGD/KLA integrated lipopeptide
A lipopeptide and resin technology, applied in the field of anticancer lipopeptide preparation, can solve the problems of easy hydrolysis by protease, restricted development, low cell membrane permeability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Preparation method of anticancer lipopeptide
[0022] The synthesis of the anticancer lipopeptide of the present invention is carried out based on the Fmoc-protected solid-phase peptide synthesis method. The sequence of anticancer lipopeptide is C 8 H 15 O-Lys-Ala-Leu-Lys-Ala-Leu-Lys-Lys-Ala-Leu-Lys-Ala-Leu-Lys-Asp-Gly-Arg, the specific synthesis steps are as follows:
[0023] (1) Fmoc-Lys(Boc)-Ala-Leu-Lys(Boc)-Ala-Leu-Lys(Boc)-Lys(Boc)-Ala-Leu-Lys(Boc)-Ala-Leu-Lys(Boc) - Synthesis of Asp(OtBu)-Gly-Arg(Pbf)-Wang Resin;
[0024] Fmoc-Lys(Boc)-Ala-Leu-Lys(Boc)-Ala-Leu-Lys(Boc)-Lys(Boc)-Ala-Leu-Lys(Boc)-Ala-Leu- Lys(Boc)-Asp(OtBu)-Gly-Arg(Pbf)-Wang Resin sample; use DMF to swell Fmoc-Arg(Pbf)-Wang Resin for 30min; take a small amount of resin for ninhydrin detection, if no If the color changes, add a DMF solution containing 20% piperidine to react for 30 minutes to remove the Fmoc protecting group; rinse the resin with DMF, DCM, and DMF in turn, and then ...
Embodiment 2
[0027]Example 2: In vitro anticancer activity assay of anticancer lipopeptides
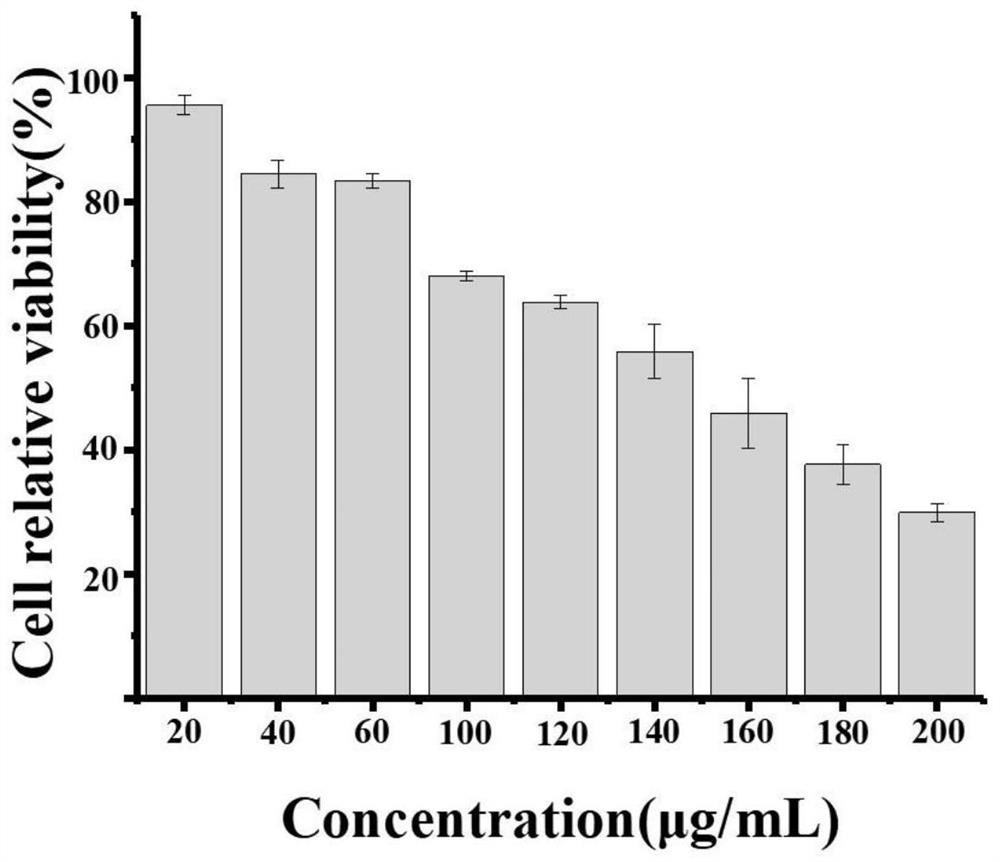
[0028] The in vitro anticancer activity of anticancer lipopeptides was tested by MTT assay. Select H22 cells in logarithmic growth phase, inoculate H22 cells into 96-well plates, and then put them in 5% CO. 2 , 37 ℃ incubator for cultivation, use medium to prepare lipopeptide into different concentrations, the concentration is set to 0 μg / mL, 20 μg / mL, 40 μg / mL, 60 μg / mL, 100 μg / mL, 120 μg / mL, 140 μg / mL, 160 μg / mL, 180 μg / mL, 200 μg / mL. 100 μL of drug-containing medium was added to each well, and 5 replicate wells were set for each concentration to reduce the influence of errors caused by experimental chance. After co-culturing the cells and the drug for 24 h, add another 20 μL (5 mg·mL) to each well. -1 ) MTT solution, placed in a cell incubator for 4 h. After 4 h, the supernatant was discarded, 150 μL of DMSO solution was added to each well, and the 96-well plate was protected from light and...
Embodiment 3
[0029] Example 3: Secondary structure determination of anticancer lipopeptides
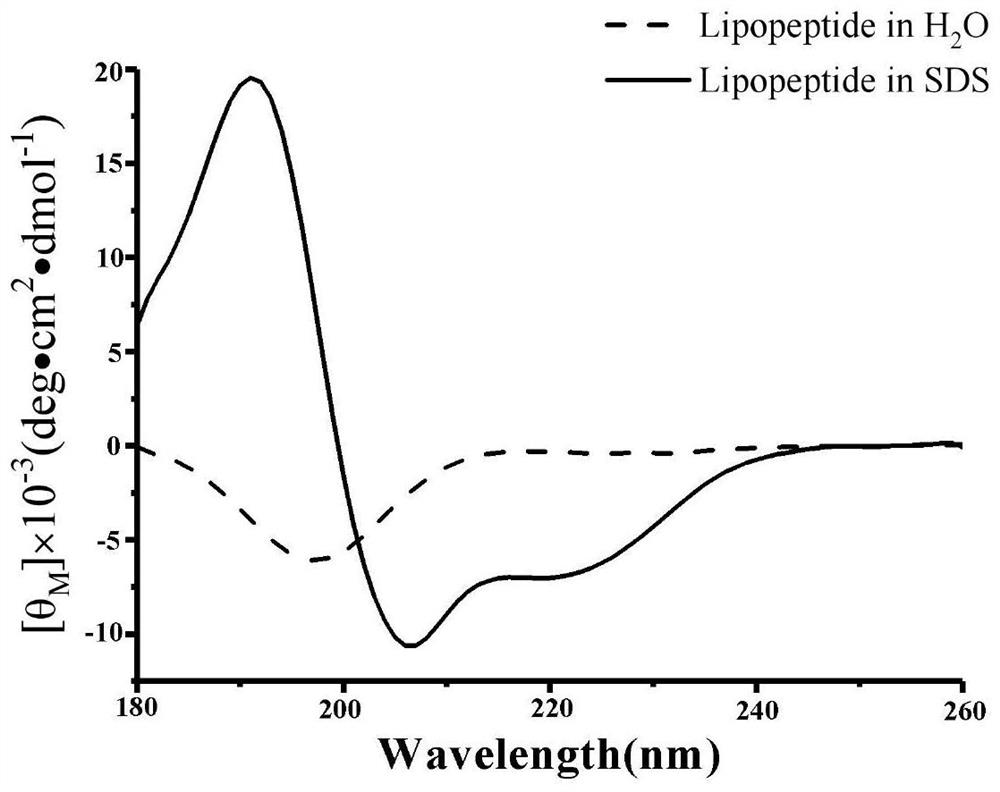
[0030] The secondary structure of lipopeptide in water and SDS solution was detected by circular dichroism (CD). The lipopeptide was dissolved in pure water and a 30 mM SDS solution to form a 150 μM lipopeptide solution, respectively. The wavelength setting range is 180-260nm, and the optical path selection is 0.5mm. The SDS solution environment can simulate the surface environment of the cancer cell membrane, so that the secondary structure changes when the lipopeptide binds to the cancer cell membrane can be observed. The data is transformed by the following formula:
[0031] θ M =θobs×1000 / cln
[0032] θ M is the average residue ellipticity (deg cm 2 ·dmol -1 ), θobs is the ellipticity (mdeg) observed at a given wavelength, c is the sample concentration (mM), l is the optical path (mm), and n is the number of peptide residues.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com