Homologous system model method for quantitatively detecting bacterial endotoxin of blood
A technology for bacterial endotoxin and quantitative detection, which can be used in measurement devices, biological tests, material inspection products, etc., and can solve problems such as differences in reaction trends and irrationality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
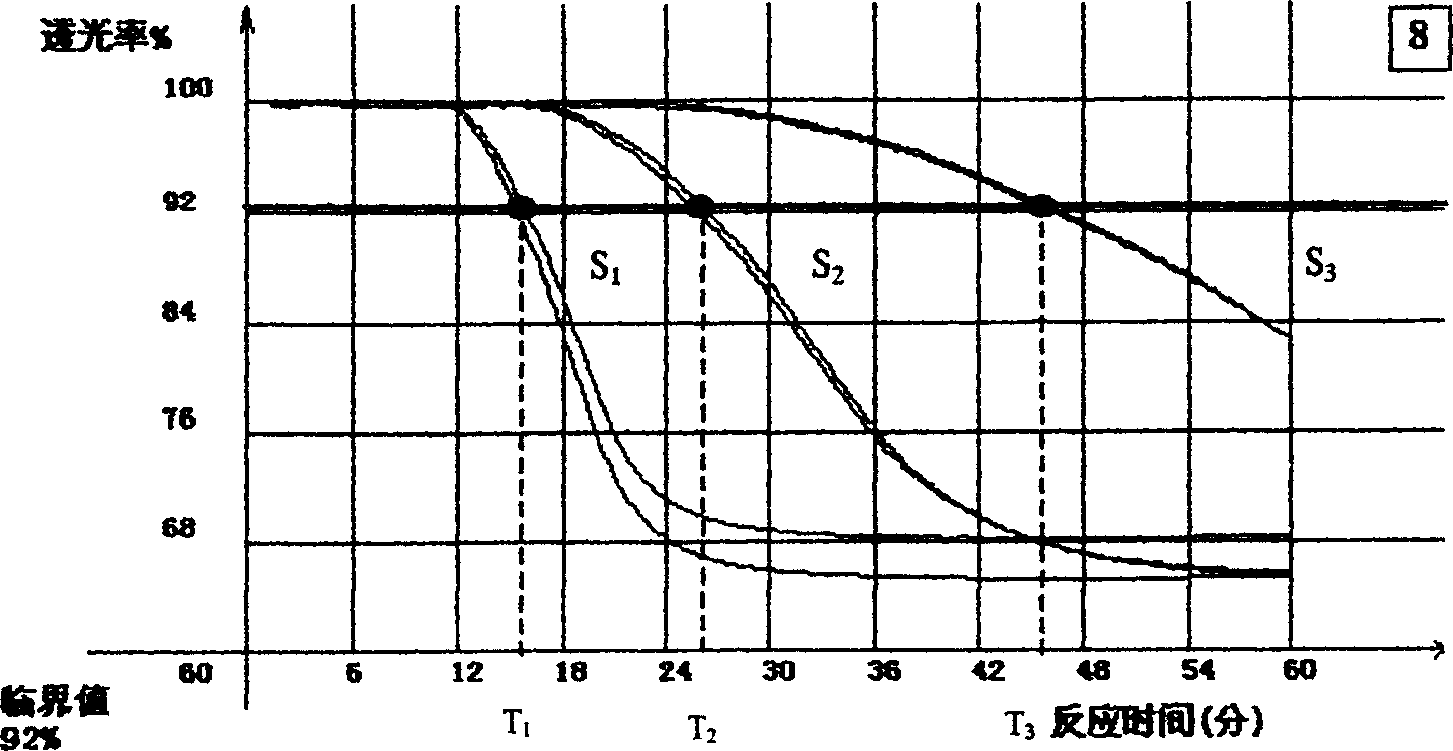
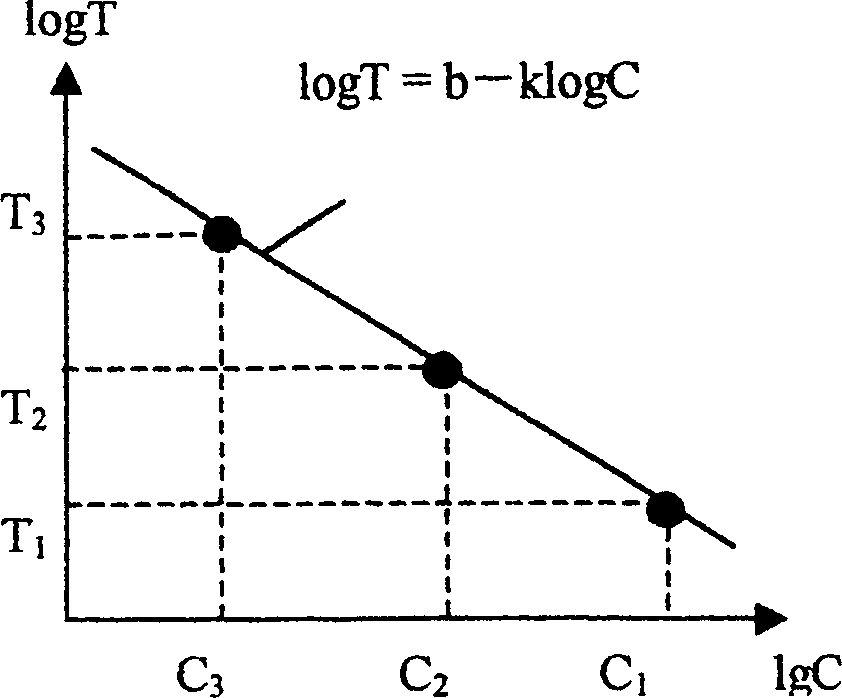
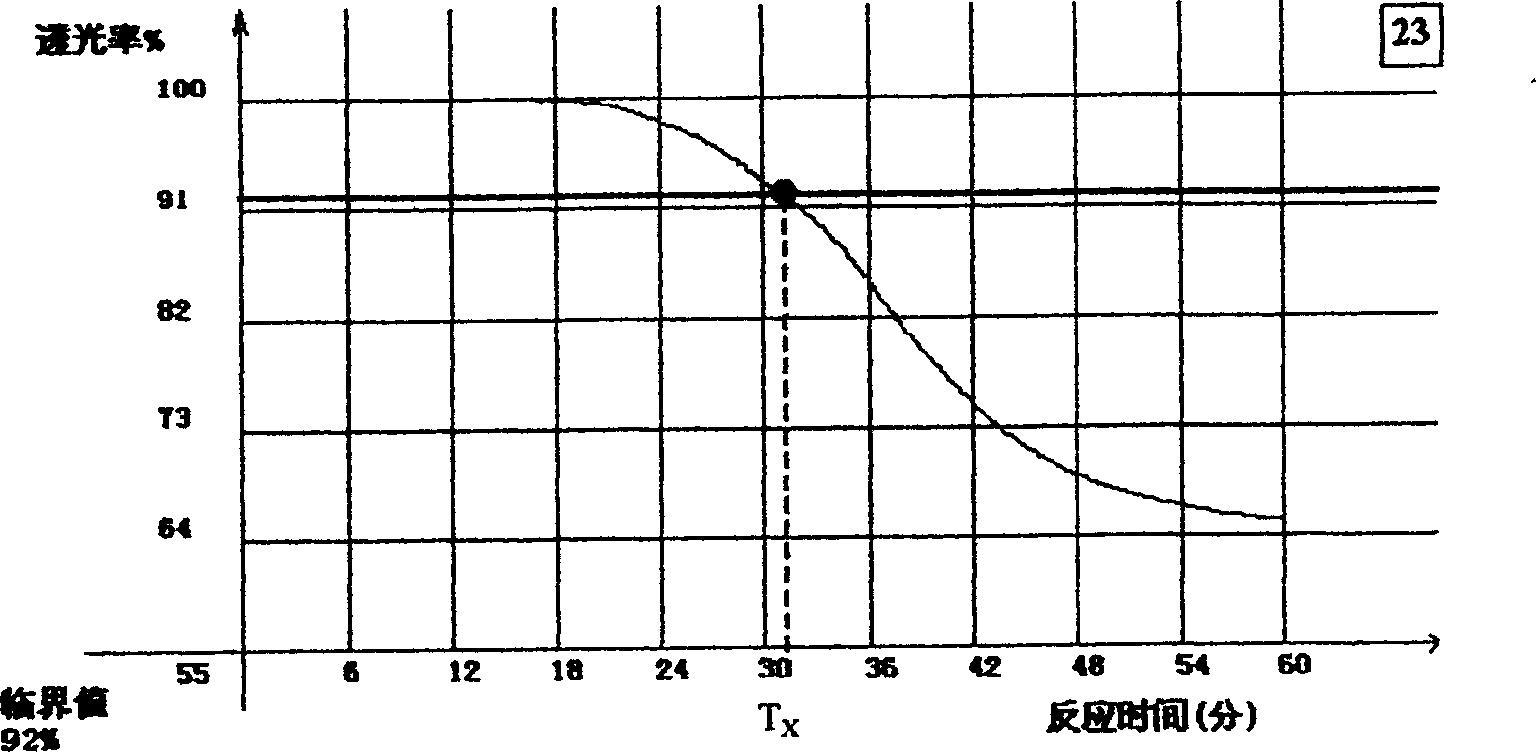
[0052] 1. The steps to establish the same system model are as follows:
[0053] a) Determine the standard endotoxin concentration required to establish the standard curve. e.g.E 0.05 ,E 0.01 and E 0 .002.
[0054] b) Take a bottle (bottle) of standard endotoxin (RSE, ISE, NSE or CSE), reconstitute and vortex according to the instructions for use and prepare for use.
[0055] c) Determine the detection concentration of the sample. For example, the detection concentration of blood samples is 1:10 dilution (S 10 ).
[0056] d) Dilute the standard endotoxin to the required concentration using the sample of detection concentration. For example, the S of the dynamic time nephelometric method 10 E. 0.05 , S 10 E. 0.01 and S 10 E. 0.002 . It is also possible to use the mutual release of the sample (S) and the standard endotoxin to obtain a test concentration sample containing the desired concentration of endotoxin. In addition, a test concentration sample S without stan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com