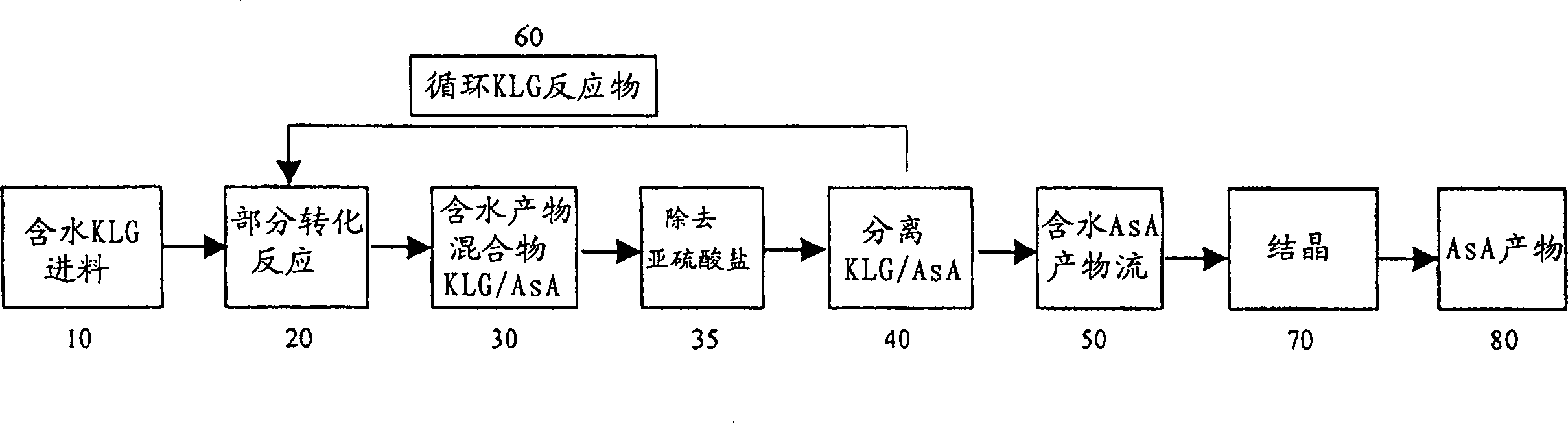
Process for producing ascorbic acid in presence of sulfite
A technology of ascorbic acid and sulfite, applied in the direction of organic chemistry, organic chemistry, chemical recycling, etc., can solve problems such as high carbon and unrealistic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Examples 1-3: Examples 1-3 (Table 1) illustrate the effect of bisulfite additive concentration on the conversion of 2-keto-L-gulonic acid to ascorbic acid and the selectivity of the reaction. In Example 1, four samples were heated at 120°C for 2 hours, and each sample contained a 10 wt% solution of 2-keto-L-gulonic acid (based on anhydride) without any sulfite, on ice After cooling in a bath, analysis of 2-keto-L-gulonic acid and ascorbic acid was performed by high performance liquid chromatography (HPLC). In Examples 2 and 3, four sodium bisulfite (NaHSO 3 ) or 0.13wt% sodium bisulfite (NaHSO 3 ) in a 10 wt% solution of 2-keto-L-gulonic acid (anhydride based) was treated as described in Example 1. The average percent conversion of 2-keto-L-gulonic acid and percent selectivity to ascorbic acid for the four samples used in each example were calculated and listed in Table 1. It can be seen that increasing sulfite does not decrease selectivity, and in fact increases sel...
Embodiment 9-23
[0115] Examples 9-23: Examples 9-23 illustrate the percent selectivity and solution color of the concentration of sulfurous acid for the conversion of 2-keto-L-gulonic acid to ascorbic acid relative to the Reference Example in which no sulfurous acid was added. influences. The reactions were carried out continuously in 1 / 8" O.D. x 50' long (30 mL volume) Teflon tubular reactors at temperatures of 120, 150 and 180°C under a total pressure of 165 psig The contact time for a continuous reactor system is equal to the reactor volume divided by the volume flow rate of the feed, and is similar to the reaction time in batch experiments.
[0116] The results are shown in Table 3. The reduced color of the sulfurous acid-treated solution was measured by absorbance at 450 nm. In all cases, an increase in color was observed upon conversion and was expressed as an absolute increase in absorbance. The difference in absorbance of the feed and product solutions can be expressed by comparing...
Embodiment 24
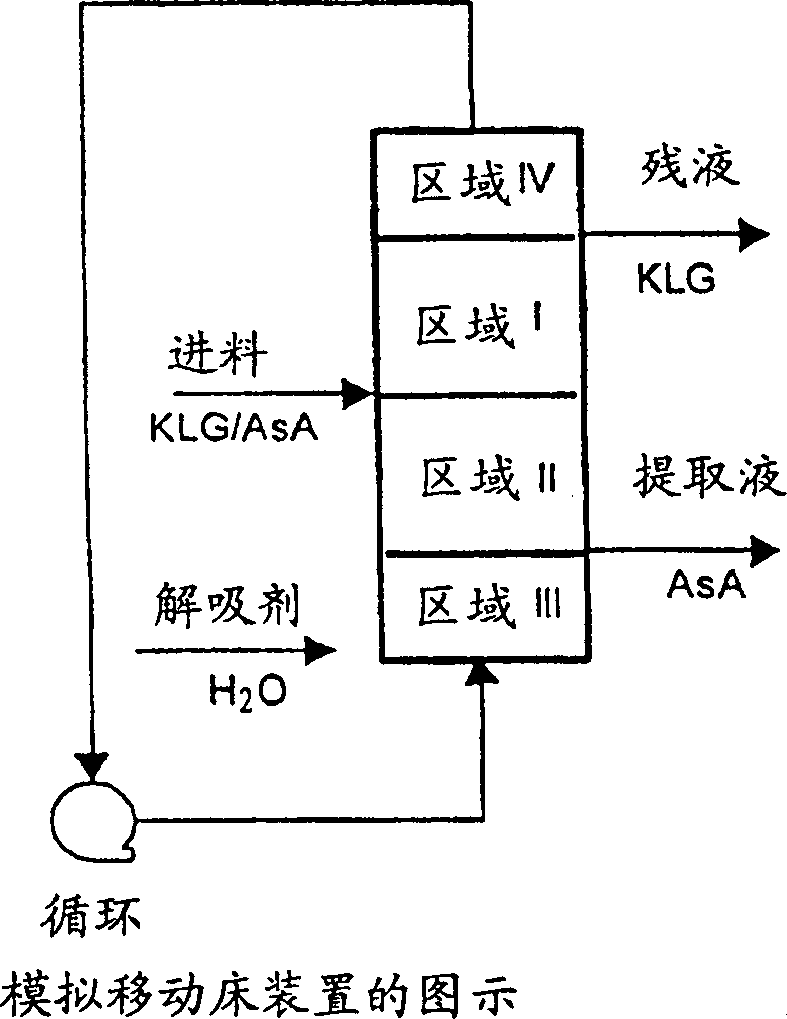
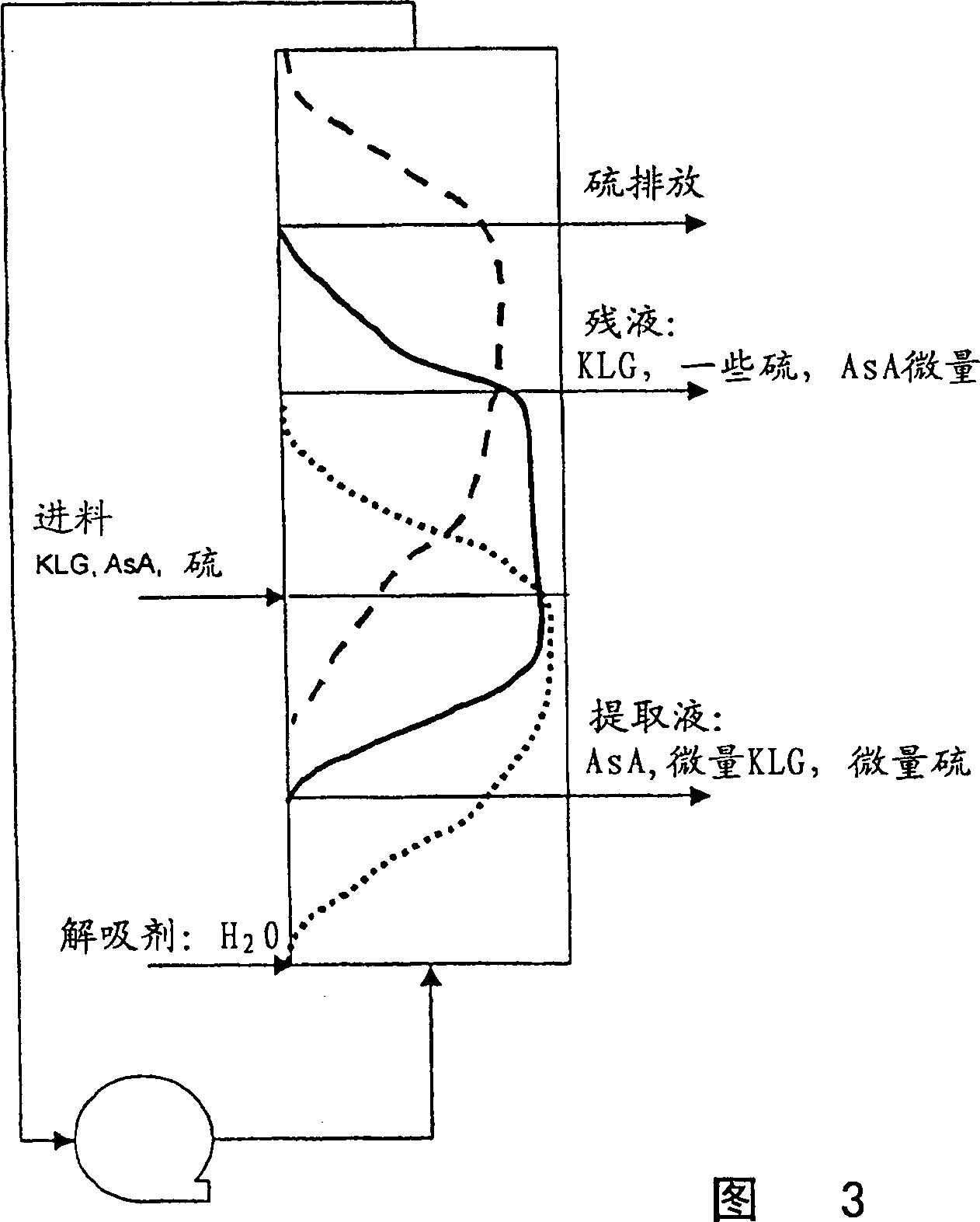
[0117] Example 24: Example 24 illustrates the separation of a solution comprising a mixture of sulfurous acid, 2-keto-L-gulonic acid and L-ascorbic acid by ion exclusion in a pulse test. A feed mixture of 0.2 column bed volumes was injected into the column packed with ion exclusion resin, the mixture was composed of 15% 2-keto-L-gulonic acid, 15% L-ascorbic acid and sulfurous acid (5.4 wt%) composition. The feed mixture was eluted with water. refer to Figure 5 , showing the peak-to-peak distances of sulfur species (S), 2-keto-L-gulonic acid (KLG) and L-ascorbic acid (AsA). Indicates that the sulfur species, 2-keto-L-gulonic acid and L-ascorbic acid peaks can be distinguished by 0.1 bed volume. This spacing in the pulse test demonstrates the feasibility of separating the L-ascorbate sulfur species, 2-keto-L-gulonic acid, in the SMB setup.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com