Uses for anti-malarial therapeutic agents
A kind of use and drug technology, applied in the field of treating colds and hydroxychloroquine, can solve the problems of no effective antiviral compounds for viral infection, no vaccines, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
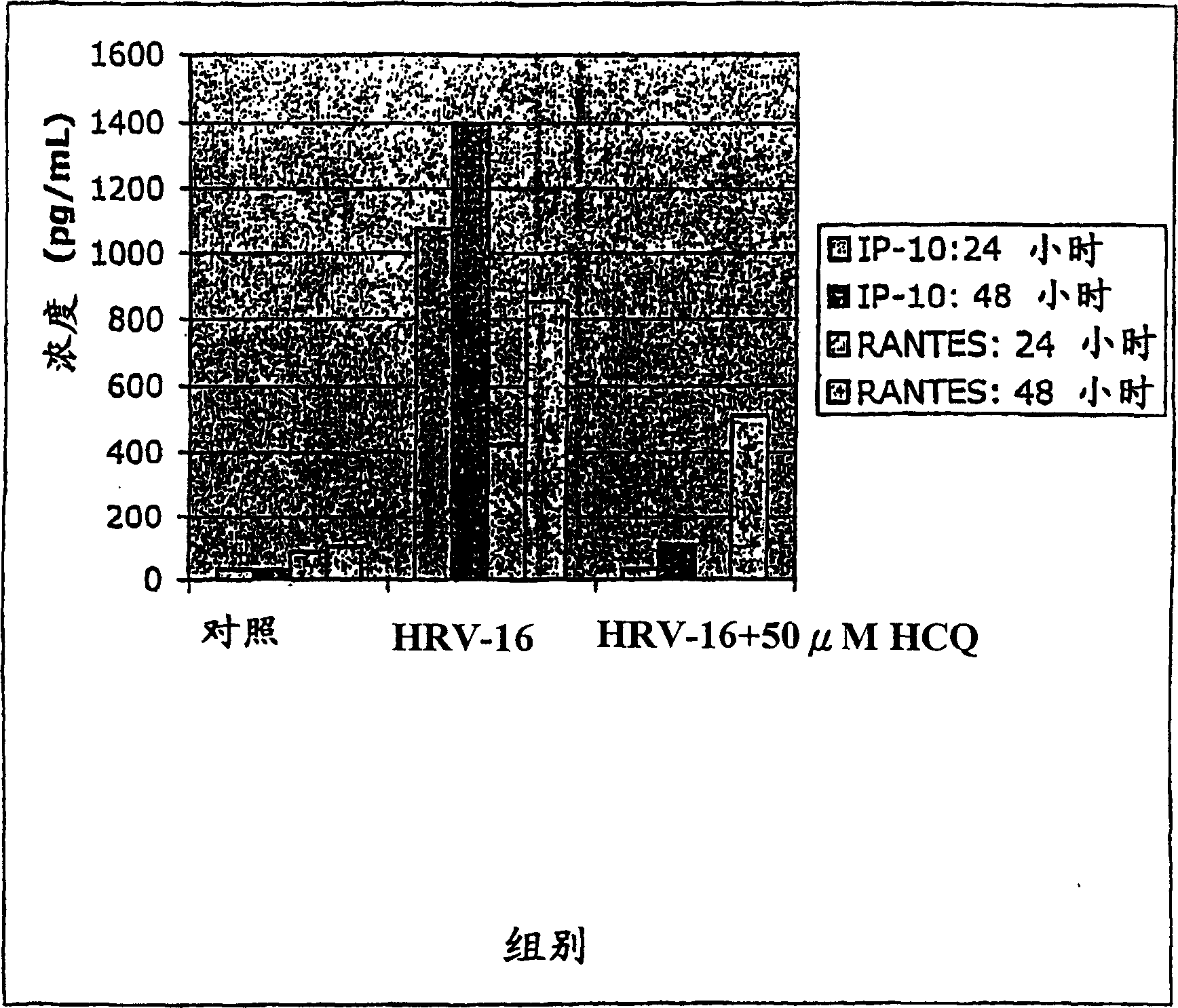
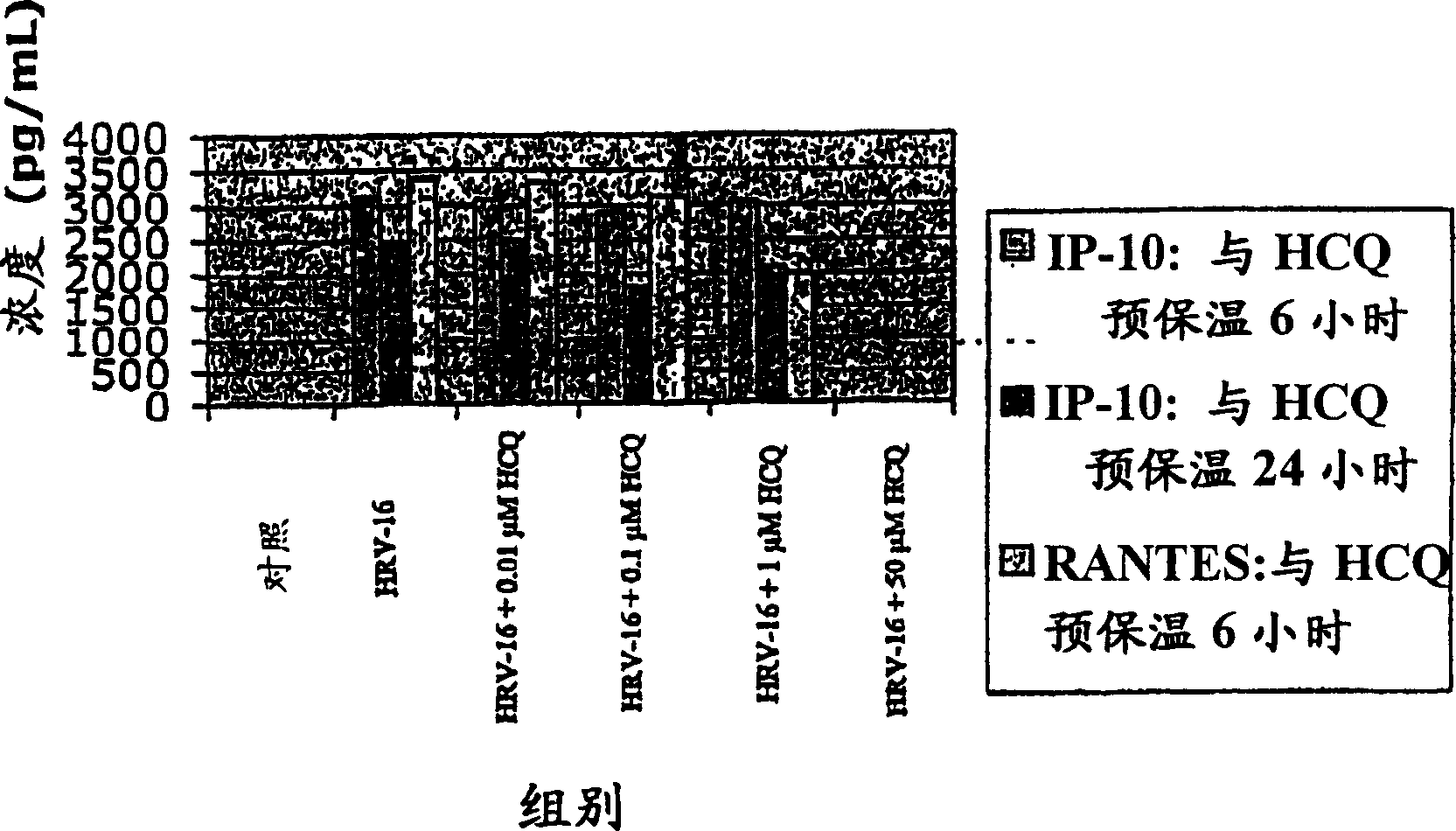
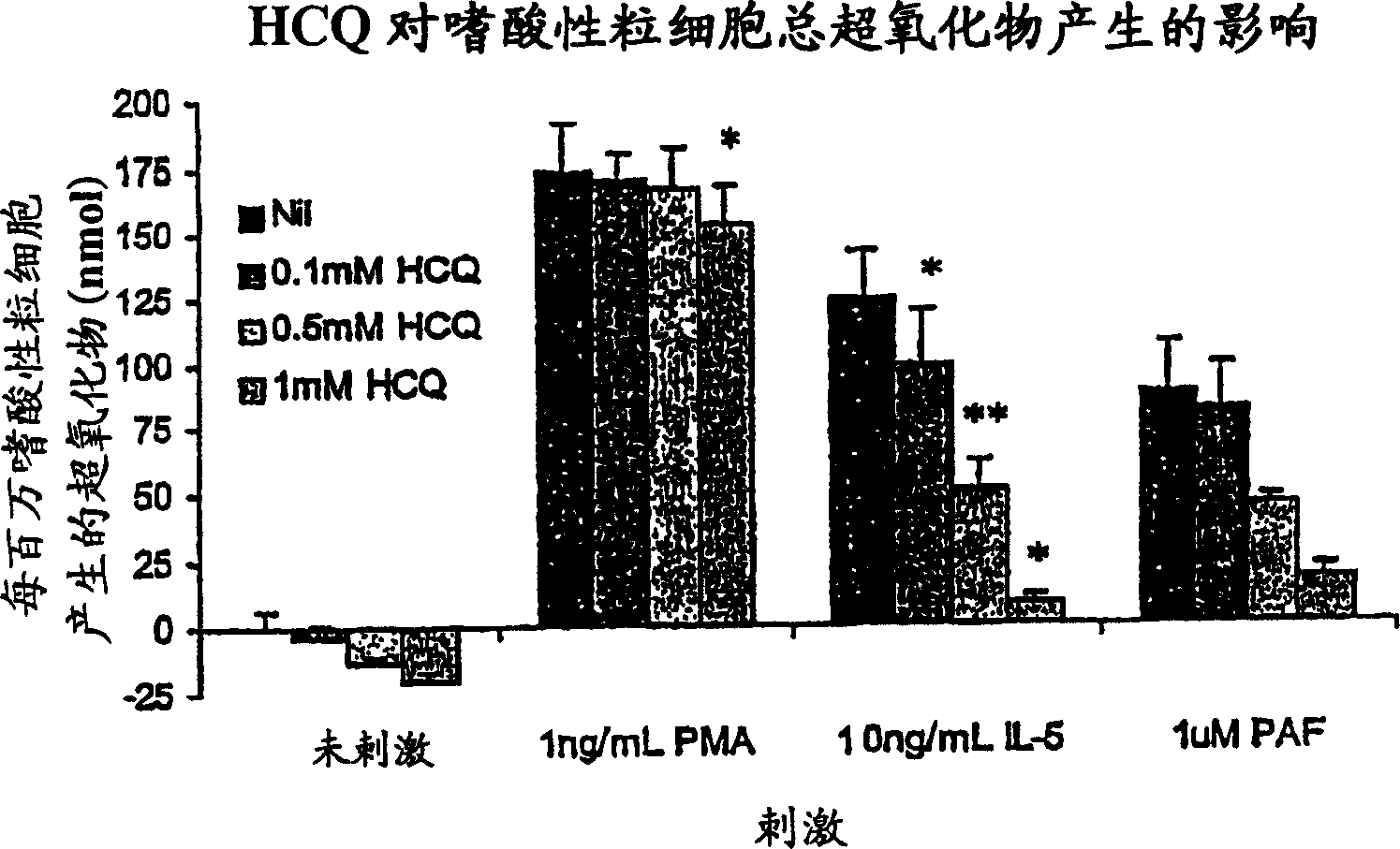
[0031] The present inventors have discovered that antimalarial compounds, most precisely antimalarial compounds including quinolines, have the ability to:
[0032] 1] Inhibition of viral infection of airway epithelial cells by rhinoviruses, adenoviruses, human coronaviruses and influenza viruses by changing the pH of entering vesicles and by reducing the expression of rhinovirus cell surface receptor ICAM-1;
[0033] 2] abolishes the effect of symptom-causing pro-inflammatory chemokines from rhinovirus-infected epithelial cells; and
[0034] 3] Inhibit the production of inflammatory mediators by leukocytes recruited to the site of viral inflammation.
[0035] The present inventors have discovered that said antimalarial compounds are most effective in treating or preventing viral infections when administered by organ-targeted delivery. The term "organ-targeted delivery" as used herein refers to direct administration to a virus-infected organ. Viral infections that cause colds...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com