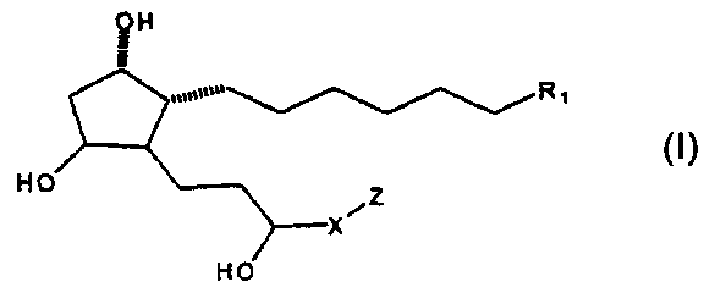
C16 unsaturated FP-selective prostaglandins analogs
A kind of technology selected from, compound, be applied in described new prostaglandin F analog, new prostaglandin F analog field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] 13,14-Dihydro-16-17-Z-didehydro-17-(2-fluorophenyl)prostaglandin F 1α preparation of a. Methyl 7-(2-oxo-4-(1,1,2,2-tetramethyl-1-silapropoxy)cyclopent-1-enyl)heptanoate (E1b):
[0059] CH 2 Cl 2 2,6-Lutidine (1.3 equivalents) was added dropwise to the solution over 15 minutes. The solution was incubated at -78°C. TBDMS triflate (Triflate) (1.2 equiv) in CH 2 Cl 2 solution. The reaction was gradually warmed to room temperature and stirred at room temperature for 15 hours. 10% aqueous HCl solution was added, and the layers were separated. use CH 2 Cl 2 The aqueous layer was extracted, and the organic layers were combined. The organic layer was washed with brine, dried (Na 2 SO 4 ), and condensed. The residue was distilled under vacuum (10 mmHg) to obtain silyl ether E1b. b. 7-(5-but-3-enyl-2-hydroxyl-4-(1,1,2,2-tetramethyl-1-silapropoxy)cyclopentyl)heptanoic acid methyl ester ( E1c):
[0060] To a THF slurry of Mg powder (2 eq.) was added crystalline iod...
Embodiment 2-17
[0067] Examples 2-17 were prepared with reference to Example 1, substituting appropriate starting materials. Those skilled in the art can appropriately change the temperature, pressure, atmosphere, solvent or sequence of the reaction. In addition, those skilled in the art can appropriately use protecting groups to block side reactions or increase yield. All of the above modifications can be readily carried out by a skilled organic chemist and, therefore, fall within the scope of the present invention.
Embodiment 2
[0069] 13,14-Dihydro-16-17-E-didehydro-17-(2-fluorophenyl)-17-trinorprostaglandin F1α
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com