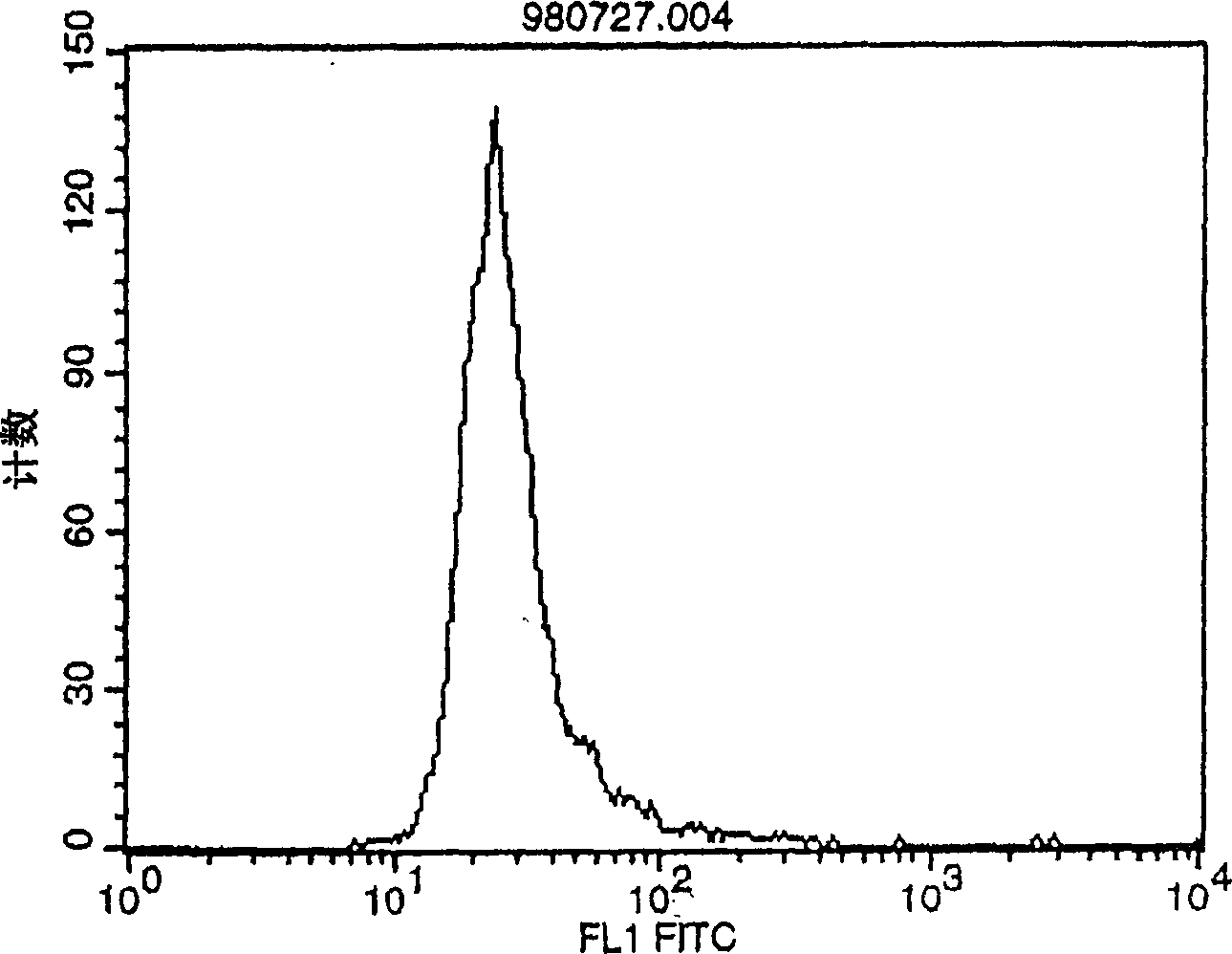
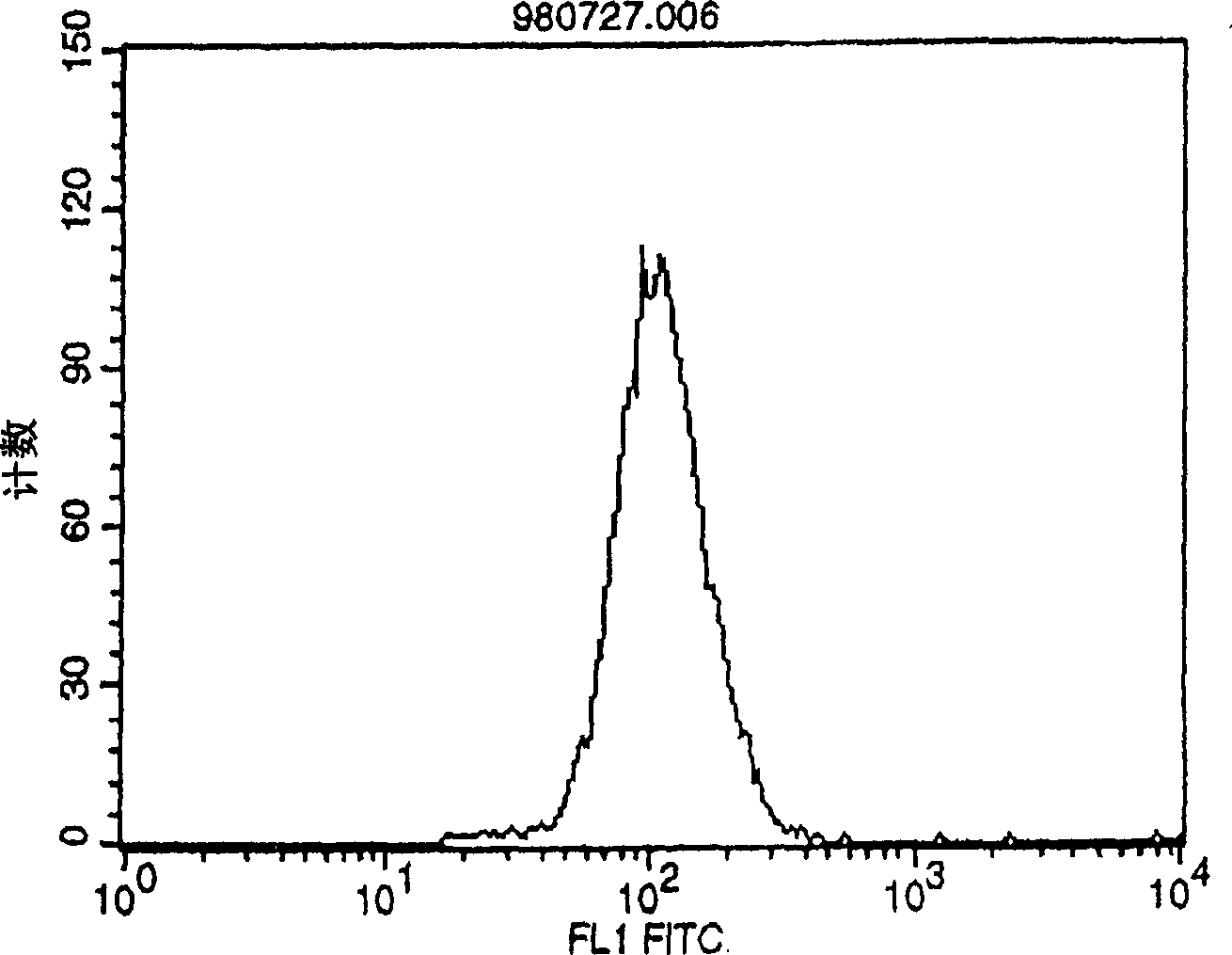
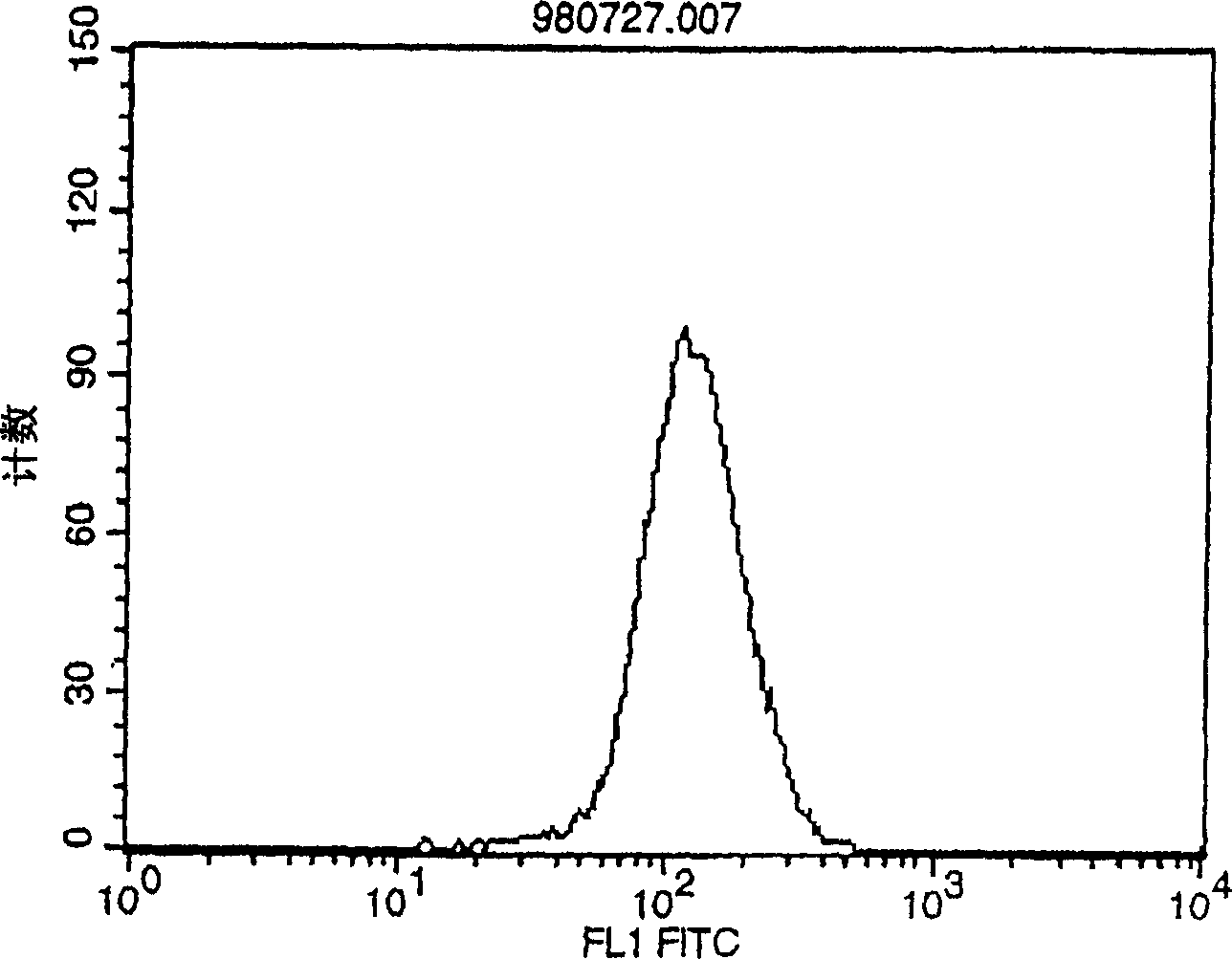
Polypeptide inducing apoptosis
A technology of apoptosis and blood cells, applied in the direction of peptides, specific peptides, peptide/protein components, etc., can solve problems such as side effects and agglutination reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] To illustrate the preparation method of the reconstituted polypeptide of the present invention, an example of single chain Fv is prepared as shown below. Mouse anti-human IAP antibodies, MABL-1 and MABL-2, were used in the example of preparing the reconstructed polypeptide. On September 11, 1997, the hybridomas MABL-1 and MABL-2 that produced these two antibodies were released internationally respectively. Deposited as FERM BP-6100 and FERM BP-6101 at the Microorganism Depository - National Institute of BioScience and human Technology, Agency of Industrial Science and Technology, Minister of International Trade and Industry Industry) (1-3 Higasi 1-Chome, Tsukuba-shi, Ibaraki-Ken, Japan). Example 1 (cloning the DNA encoding the V region of the mouse anti-human IAP monoclonal antibody)
[0069] The DNAs encoding the variable regions of the mouse anti-human IAP monoclonal antibodies MABL-1 and MABL-2 were cloned as follows. 1.1 Preparation of messenger RNA (mRNA)
[00...
Embodiment 2
[0088] A plasmid containing the gene encoding the mouse H chain V region derived from hybridoma MABL-2 was prepared from the purified DNA fragment and named pGEM-M2H. Embodiment 2 (DNA sequencing)
[0089] The nucleotide sequence of the cDNA coding region in the above plasmid was determined using Auto DNA Sequencer (Applied Biosystem) and ABI PRISM DyeTerminator Cycle Sequencing Ready Reaction Kit (Applied Biosystem) according to the manufacturer's protocol.
[0090]The plasmid pGEM-M1L contains the gene encoding the V region of the L chain of the mouse antibody MABL-1, and its nucleotide sequence is shown in SEQ ID No.5.
[0091] The plasmid pGEM-M1H contains the gene encoding the H chain V region of the mouse antibody MABL-1, and its nucleotide sequence is shown in SEQ ID No.6.
[0092] The plasmid pGEM-M2L contains the gene encoding the L chain V region of the mouse antibody MABL-2, and its nucleotide sequence is shown in SEQ ID No.7.
[0093] The plasmid pGEM-M2H contain...
Embodiment 3
[0093] The plasmid pGEM-M2H contains the gene encoding the H chain V region of the mouse antibody MABL-2, and its nucleotide sequence is shown in SEQ ID No.8. Embodiment 3 (determining CDR)
[0094] The V regions of the L chain and the H chain are generally similar in structure, and every four framework regions are connected by three hypervariable regions, ie, complementarity determining regions (CDRs). The amino acid sequences of the framework are relatively conserved, whereas those of the CDRs are extremely highly variable (Kabat E.A. et al., "Sequences of Proteins of Immunological Interest", US Dept. Health and Human Services, 1983).
[0095] Based on these facts, the amino acid sequence of the variable region of the mouse anti-human IAP monoclonal antibody was entered into the antibody amino acid sequence database established by Kabat et al., and its homology was searched. Table 1 shows the CDR regions determined by homology.
[0096] Table 1
[0097] Plasmid SEQ ID...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com