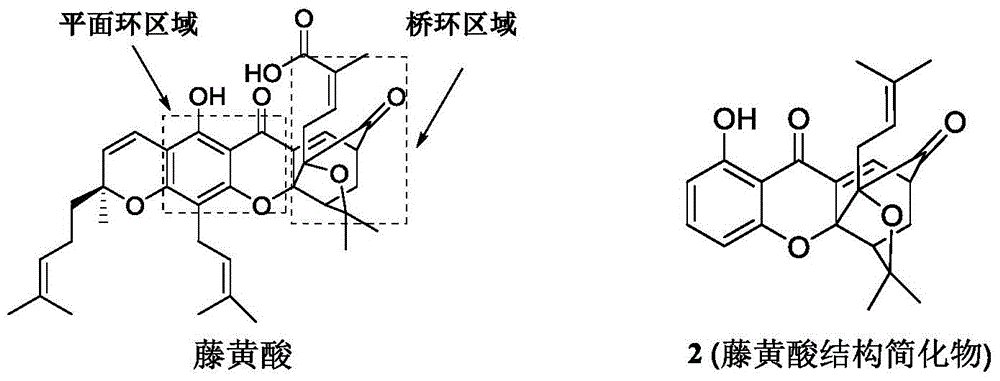
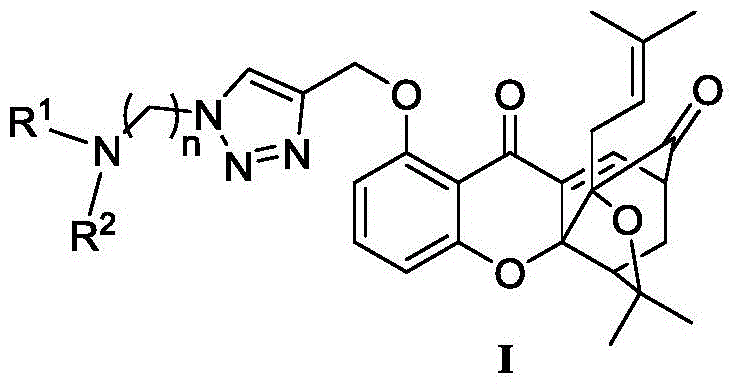
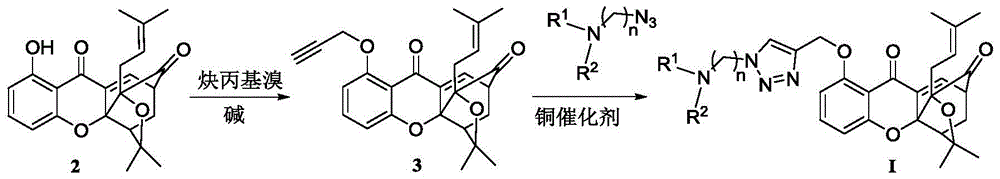
A class of garcinia triazole derivatives, preparation method and medicinal use thereof
A pharmacy and drug technology, applied in the direction of pharmaceutical formula, antineoplastic drugs, drug combinations, etc., can solve the problems of lack of druggability, water solubility, lack of hydrophilic nitrogen atoms, poor activity in vivo, etc., and achieve good antitumor activity in vivo, Good anti-tumor activity, the effect of improving cell membrane permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 8-(1-(2-(Dimethylamino)ethyl)-1H-1,2,3-triazol-4-yl)methoxy-3,3-dimethyl-1-(3- Methylbut-2-en-1-yl)-3,3a,4,5-tetrahydro-1,5-methyl-1H,7H-furo[3,4-d]xanthene-7, 13-Diketone (I-1)
[0053]
[0054] (1) Dissolve 1-hydroxy-5,6-bis(2-methylbut-3-en-2-yloxy)-9H-xanthene-9-one (compound 2) (30g, 0.105mol) In anhydrous tetrahydrofuran (400mL), cool in an ice bath to 10°C, add tert-butoxycarbonylmethylbutenol (195g, 1.05mol), and add tetrakistriphenylphosphine palladium (1.2g, 1.05mmol) under nitrogen protection , Reaction at 10°C for 10h. Air was introduced to quench the reaction, and silica gel was used to filter. The filtrate was concentrated by distillation under reduced pressure to obtain compound 3 as a yellow oil. 1 H NMR (300MHz, CDCl 3 ):δ1.56(s,6H),1.57(s,6H),2.48(s,3H),5.04(d,J=10.8Hz,1H),5.17-5.24(m,3H),5.11-6.32( m,2H),6.97(d,J=8.4Hz,1H),7.08(d,J=9.0Hz,1H),7.41(d,J=8.4Hz,1H),7.63(t,J=8.4Hz, 1H), 7.82(d, J=9.0Hz, 1H); EI-MS m / z: 422[M] + (5), 286(12), 244(...
Embodiment 2
[0057] 8-(1-(3-(Dimethylamino)propyl)-1H-1,2,3-triazol-4-yl)methoxy-3,3-dimethyl-1-(3- Methylbut-2-en-1-yl)-3,3a,4,5-tetrahydro-1,5-methyl-1H,7H-furo[3,4-d]xanthene-7, 13-Diketone (I-2)
[0058]
[0059] Compound 3 (0.6g, 1.4mmol) was dissolved in t-BuOH / H 2 In the mixed solution of O (v:v=1:1, 20mL), add the side chain azide N,N-dimethylaminoazidopropane (0.28g, 2.2mmol), sodium ascorbate (0.4mmol), five Copper sulfate water (0.01mmol), N 2 Protection, reaction at room temperature for 24h. Add 40ml of water to the reaction solution, CH 2 Cl 2 Extraction (15mL×4), combined organic layers, anhydrous NaSO 4 Dry, filter with suction, and concentrate the filtrate under reduced pressure to obtain the product as light yellow solid I-2 (0.71 g, 93%). m.p.133-136°C; 1 H NMR (300MHz, CDCl 3 ):δ1.08(s,3H),1.25-1.29(m,4H),1.37(s,3H),1.69(s,3H),2.15-2.25(m,2H),2.28-2.41(m,8H ),2.48-2.53(m,2H),2.61(d,J=7.7Hz,2H,C 11 -H),3.43-3.47(m,1H),4.45-4.49(m,3H),5.31(s,2H),6.71(t,J=7.8H...
Embodiment 3
[0061] 8-(1-(Dimethylaminomethyl)-1H-1,2,3-triazol-4-yl)methoxy-3,3-dimethyl-1-(3-methylbutyl- 2-en-1-yl)-3,3a,4,5-tetrahydro-1,5-methyl-1H,7H-furo[3,4-d]xanthene-7,13-dione (I-3)
[0062]
[0063] Compound 3 (0.6g, 1.4mmol) was dissolved in t-BuOH / H 2 In the mixed solution of O (v:v=1:1, 2mL), add the side chain azide N,N-dimethylaminoazomethane (0.22g, 2.2mmol), sodium ascorbate (0.4mmol), five Copper sulfate water (0.01mmol), N 2 protected and reacted overnight at room temperature. Add 20 mL of water to the reaction solution, CH 2 Cl 2 Extraction (15mL×4), combined organic layers, anhydrous NaSO 4 Dry, filter with suction, and concentrate the filtrate under reduced pressure to obtain the product as light yellow solid I-3 (0.70 g, 93%). m.p.146-148°C; 1 H NMR (300MHz, CDCl 3 ): δ1.01(s,3H),1.18-1.24(m,4H),1.32(s,3H),1.65(s,3H),2.22-2.27(m,7H),2.33(d,J=9.5 Hz,1H),2.54(d,J=7.8Hz,2H),,4.37-4.46(m,1H),4.49(s,1H),4.52(s,2H),5.26(s,2H),6.62- 6.71(m,2H),7.21-7.24(m,1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com