Hepatocellular carcinoma-targeted paclitaxel galactosamine conjugate, nanoparticles as well as preparation method and application thereof
A technology of galactosamine and paclitaxel, applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of limiting the clinical transformation and wide application of nano-drugs, avoid toxic side effects, and achieve good in vivo tumor inhibition effect. , uniform size effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
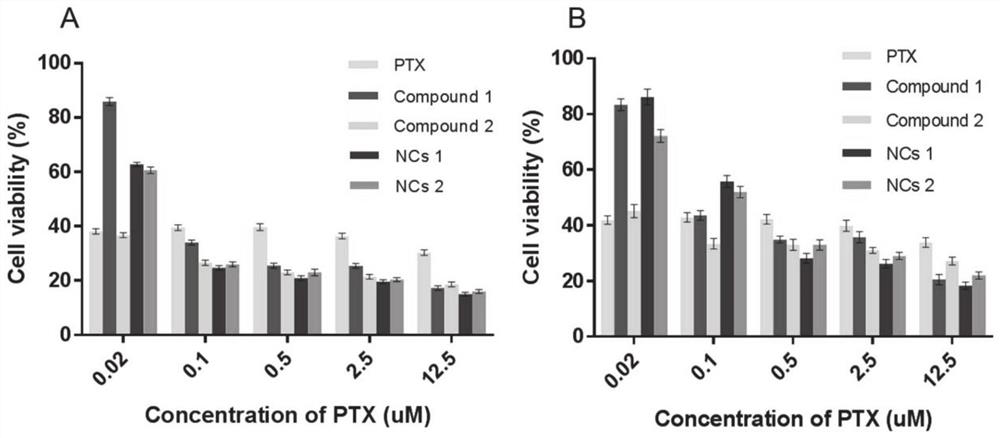
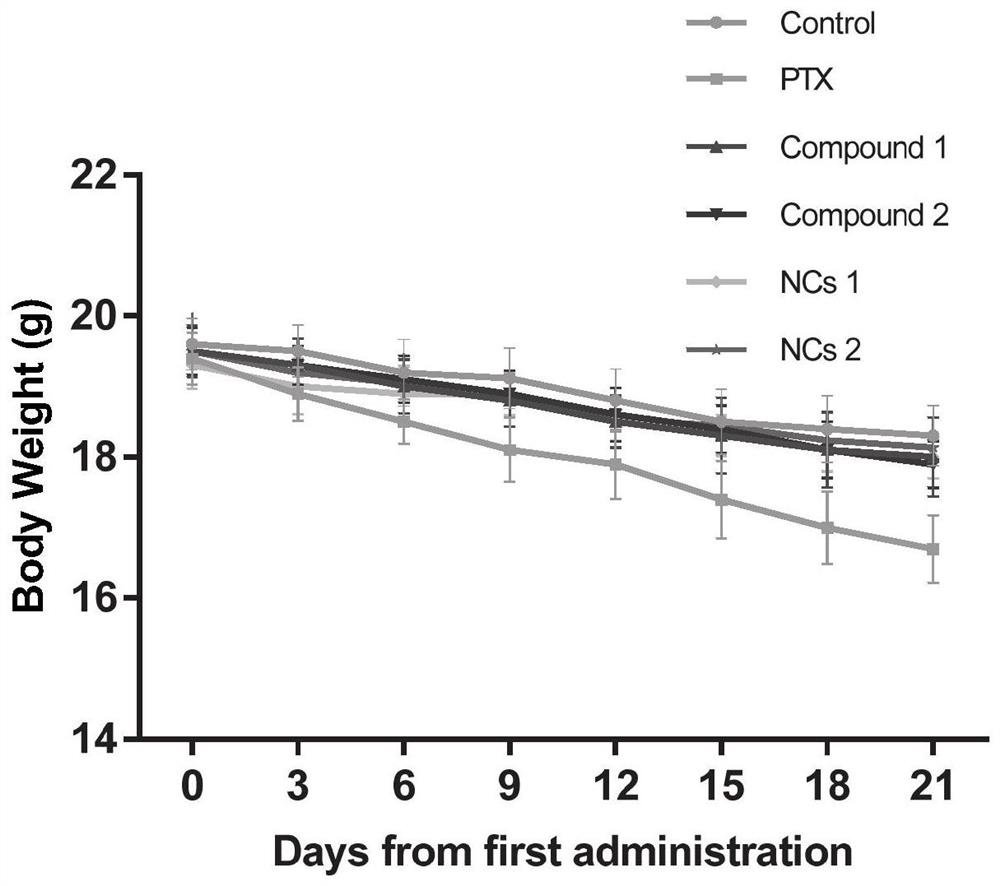
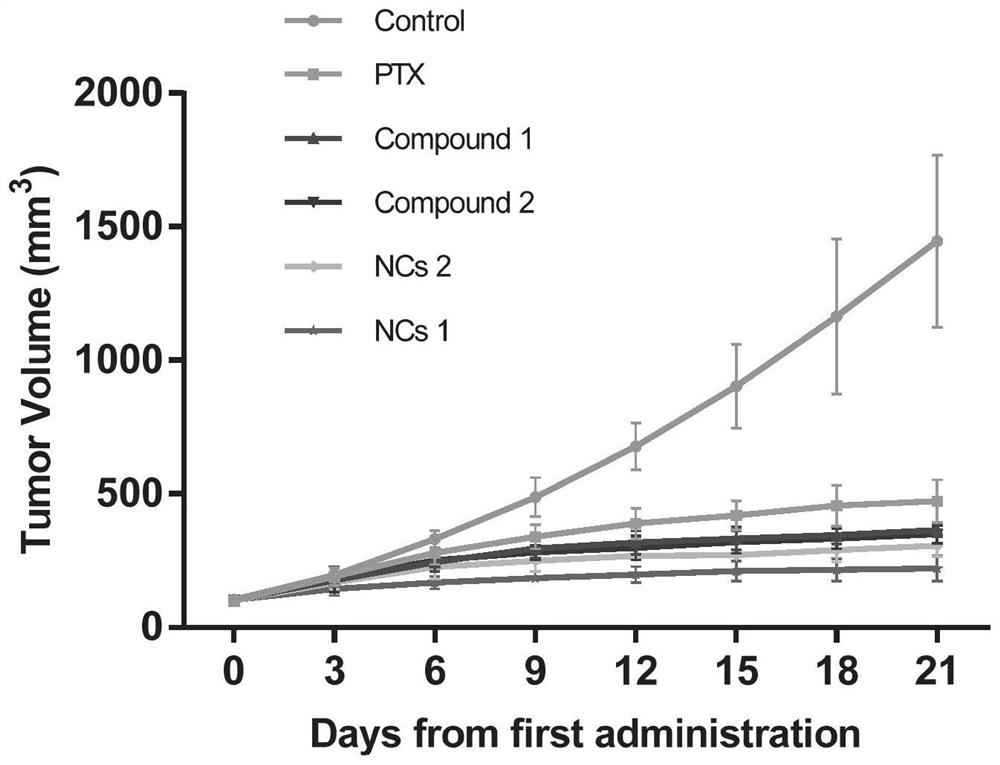
Image
Examples
preparation example Construction
[0047] In yet another specific embodiment of the present invention, a preparation method of the above paclitaxel-galactosamine conjugate is provided, the preparation method comprising: using pentaerythritol as the basis, and linking the liver-targeting ligand and the anticancer drug with a succinyl structural unit. Benzaldehyde protects the two hydroxyl groups of pentaerythritol, and then introduces succinyl paclitaxel and stearic acid, followed by FeCl 3 Carrying out deprotection and succinic anhydride reaction to introduce carboxyl groups, and finally undergoing amidation reaction with two galactosamines to obtain paclitaxel galactosamine conjugate shown in formula 1;
[0048] A hydroxyl group of pentaerythritol undergoes an esterification reaction with succinyl-paclitaxel, then reacts with succinic anhydride to introduce a carboxyl group, and finally undergoes amidation reaction with three galactosamines to obtain the paclitaxel-galactosamine conjugate shown in formula 2.
...
Embodiment 1
[0095] Embodiment 1. Preparation of intermediate formula 3 compound
[0096] Succinyl paclitaxel (0.477g, 0.50mmol) and pentaerythritol monobenzaldehyde (0.113g, 0.50mmol), DMAP (12.2mg, 0.1mmol) and EDC (0.192g, 1mmol) were dissolved in 15ml of dichloromethane, room temperature The reaction was stirred for 20 hours, and the solvent was distilled off under reduced pressure. The resulting crude product was separated and purified by silica gel column chromatography to obtain 0.278 g of the compound of formula 3, with a yield of 48%. ESI-MSm / z:1195.48[M+Cl] - .
Embodiment 2
[0097] Embodiment 2. Preparation of intermediate formula 4 compound
[0098] Formula 3 compound (0.232g, 0.2mmol) and 0.16g triethylamine are dissolved in 4 milliliters of dichloromethane, dropwise add the dichloromethane solution of 2 milliliters of stearyl chloride (0.3mmol), react at room temperature for 20 hours, remove by distillation under reduced pressure Solvent, the resulting crude product was separated and purified by silica gel column chromatography to obtain 0.151 g of the compound of formula 4 with a yield of 53%. ESI-MS m / z:1448.74[M+Na] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com