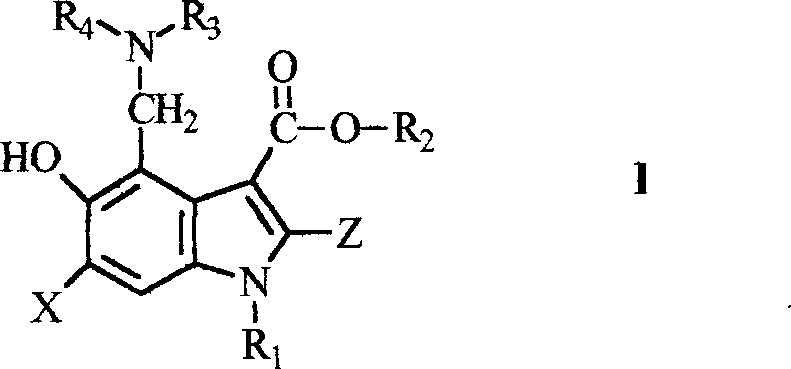
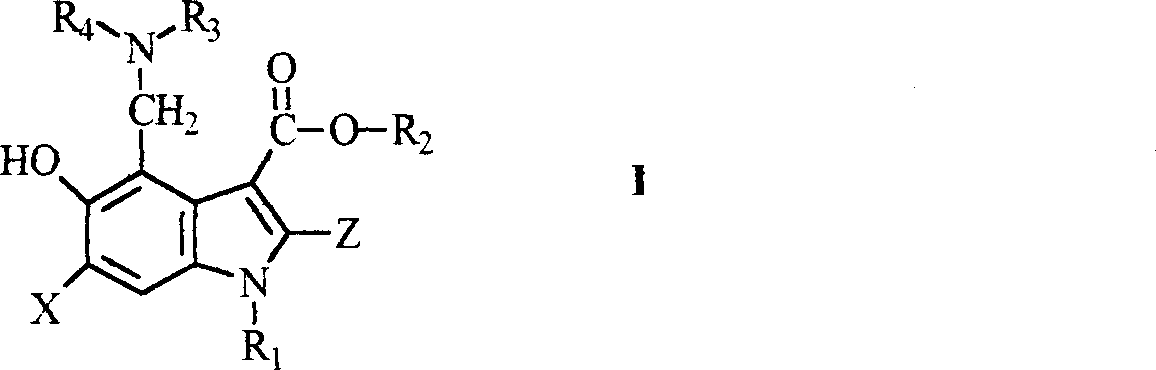
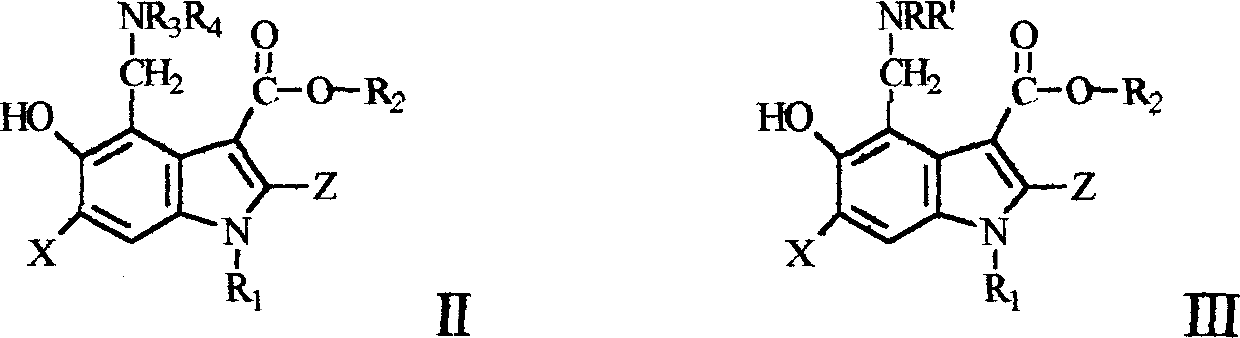Novel 5-hydroxy-3-carboxylate indoles derivant and method for preparing the same
A derivative and indole technology, applied in the field of novel 5-hydroxy 3-carboxylate indole derivatives and its preparation, can solve the problems of influenza vaccine failure, drug tolerance and side effects, and anti-influenza drug types and limited number of issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] 6-Bromo-4-(diethylamino)methyl-5-hydroxy-1-methyl-2-[(phenyl)thio]methyl-1H-indole-3-carboxylic acid ethyl ester salt salt
[0096] Step A: Preparation of ethyl 3-methylamino-3-butenoate (1)
[0097] Under stirring with slight heat, 274ml (1.4mol) of methylamine solution was added dropwise to 300ml of 50% sodium hydroxide solution, and the generated methylamine gas was introduced into 169ml (1.3mol) of ethyl acetoacetate, the reaction was exothermic, and stirred , cooling the reaction solution in a water bath, and maintaining the reaction temperature at 35-40° C. until the reaction solution becomes cloudy to obtain a light green turbid solution, stop aeration, and stir overnight at room temperature. The next day, stir and pump air for 2 hours, add 300ml of diethyl ether to separate layers, wash the organic layer with water until the pH is 8, dry over anhydrous sodium sulfate, and concentrate the organic layer under reduced pressure to obtain 180.6g of transparent liqui...
Embodiment 2
[0109] 6-Bromo-4-(dimethylamino)methyl-5-hydroxy-1-ethyl-2-[(4-isopropylphenyl)thio]methyl-1H-indole-3- ethyl carboxylate phosphate
[0110] 1 H-NMR (DMSO): 1.25~1.38(m, 12H), 2.49(s, 6H), 2.95(m, 1H), 4.18~4.34(s, m, 6H), 4.69(s, 2H), 7.26( d, 2H), 7.36(d, 2H), 7.38(s, 1H), 7.89(s, 1H)
Embodiment 3
[0112] 6-Bromo-4-(dimethylamino)methyl-5-hydroxy-1-ethyl-2-[(4-fluorophenyl)thio]methyl-1H-indole-3-carboxylic acid Ethyl phosphate
[0113] 1 H-NMR(DMSO): 1.23(t, 3H), 1.29(t, 3H), 2.38(s, 6H), 4.11(q, 2H), 4.20~4.24(m, 4H), 4.60(s, 2H) , 7.16(m, 2H), 7.35(m, 2H), 8.01(s, 1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com