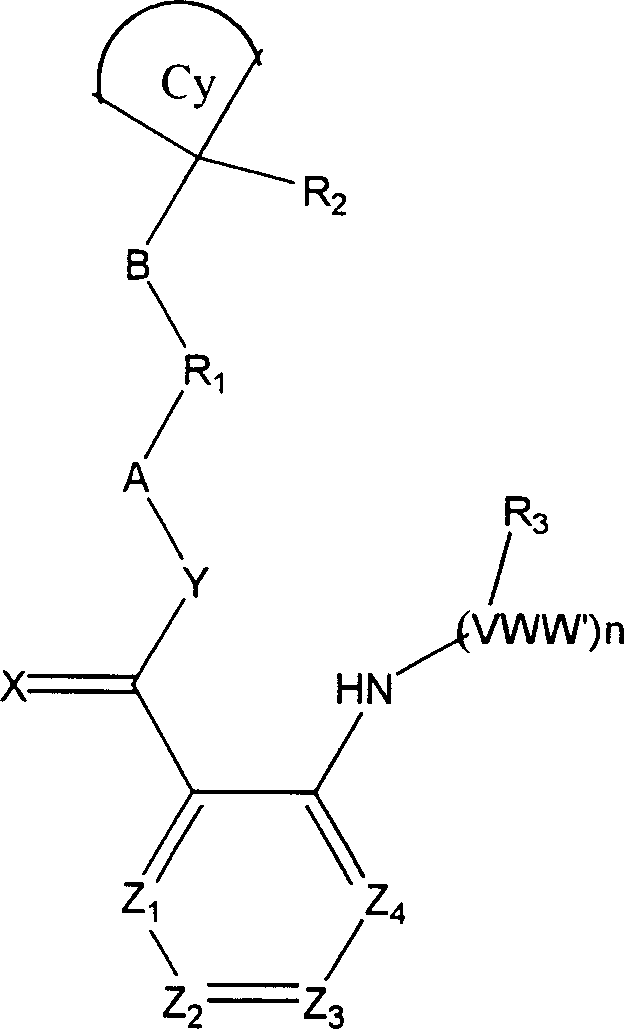
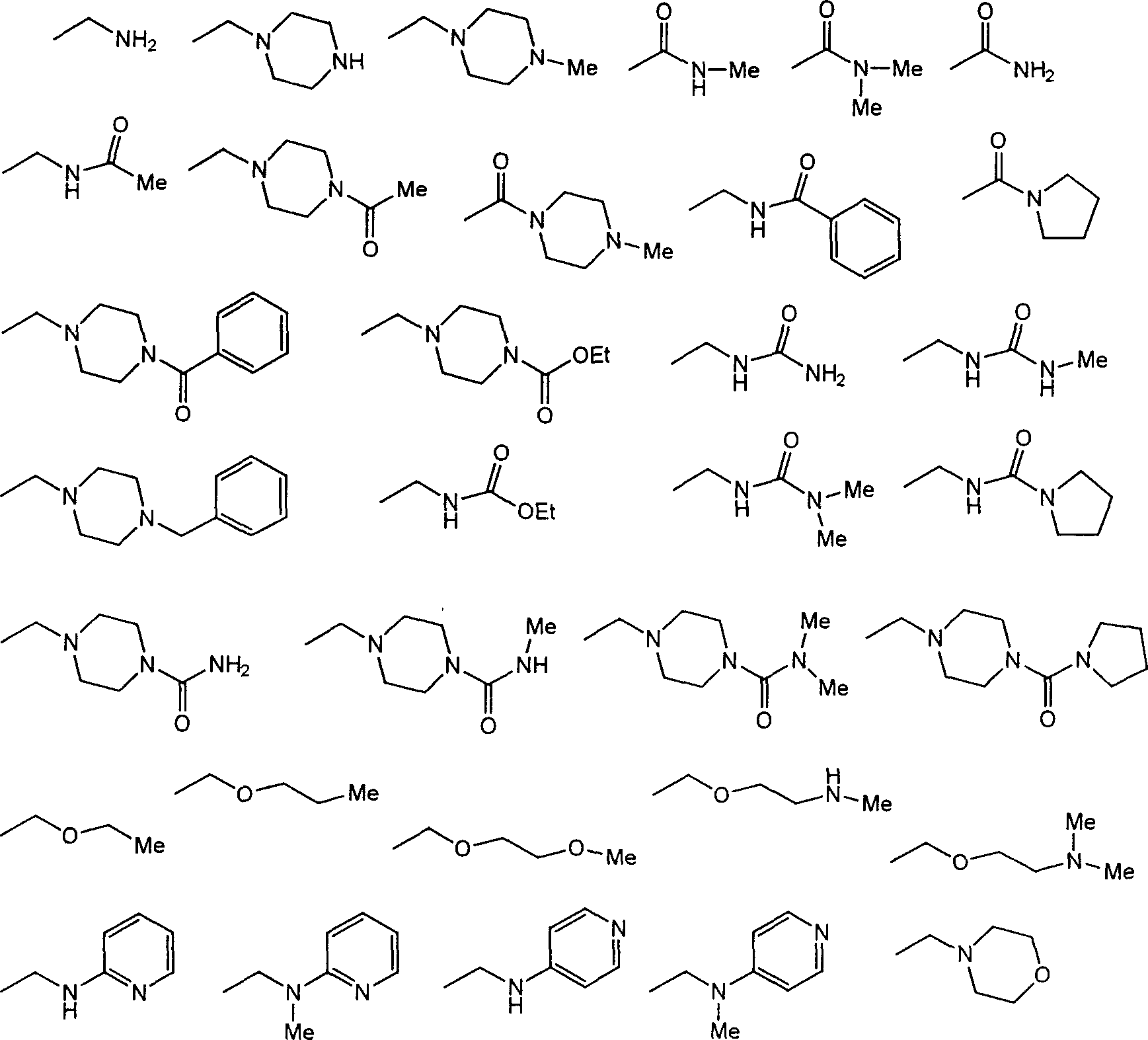
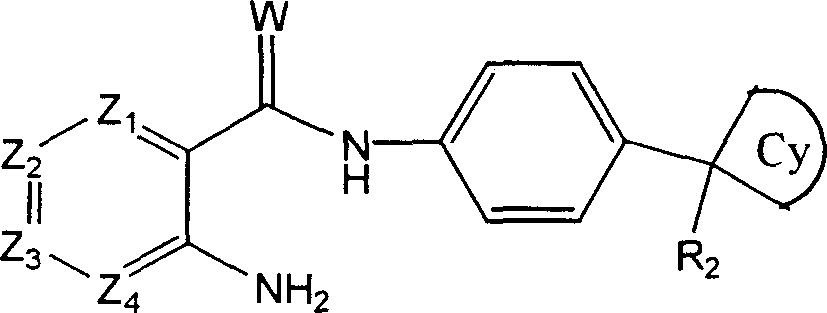
Hexavalent amino amidate derivative with function of inhibiting blood vessel growth activity
A carbamoyl, aminoacyloxy technology, applied in the field of treatment of angiogenesis or high vascular permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137] N-[4-(cyanocyclobutyl)phenyl]{2-[(4-pyridylmethyl)amino]phenyl}carboxamide
[0138] A: 1-(4-Aminophenyl)cyclobutylnitrile
[0139] Mix 1-phenylcyclobutyronitrile (5 g), acetic acid (15 ml), H 2 SO 4 (10ml) and KNO 3 (1.1 molar ratio), stirred at 0° C. for 20 minutes, then warmed to room temperature and stirred for another 2 hours. Then the mixture was poured into ice cubes and stirred until the ice cubes were completely dissolved, and a solid precipitated out, which was filtered by suction and recrystallized with ethanol to obtain 1-(4-nitrophenyl)cyclobutyronitrile. Mix 2 g of the above product with palladium-carbon (800 mg, 10%) and ethanol (100 ml), hydrogenate at normal pressure for 1 hour, then filter with suction, and evaporate the filtrate to dryness to obtain 1-(4-aminophenyl)cyclobutane base nitrile. Mass spectrum (M+1), 172, the product was not purified for use in the next step.
[0140] B. 2-Aminophenyl-N-[4-(cyanocyclobutyl)phenyl]carboxamide
[0141]...
Embodiment 2
[0145] N-[4-(cyanocyclopropyl)phenyl]{2-[(4-pyridylmethyl)amino]phenyl}carboxamide
[0146] This compound was prepared in a manner similar to that of Example 1, starting from 1-phenylcyclopropylnitrile. Mass spectrum: (M+1), 369
Embodiment 3
[0148] N-[4-(cyanocyclopentyl)phenyl]{2-[(4-pyridylmethyl)amino]phenyl}carboxamide
[0149] This compound was prepared in a manner similar to that of Example 1, starting from 1-phenylcyclopentylnitrile. Mass spectrum: (M+1), 395
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com