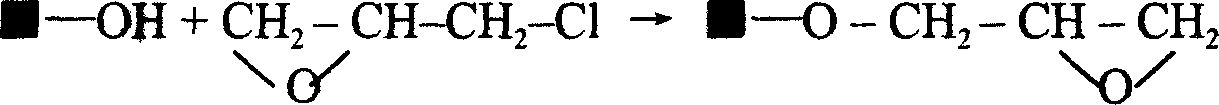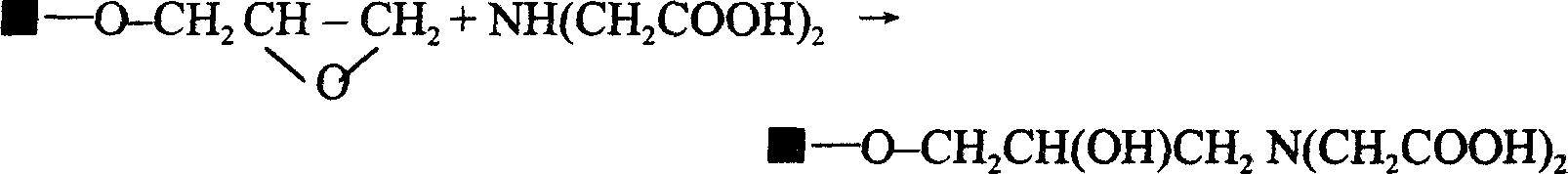Preparation method of metal chelation chromatography stuffing
A metal chelate layer, agarose gel technology, applied in the field of protein purification, can solve the problems of low flow rate of fillers, low molecular exclusion limit, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Take 40g of dried Sepharose CL-4B, put it into a 200ml beaker, add 20ml of 2M sodium hydroxide, 2ml of epichlorohydrin, and 75mg of sodium borohydride, mix well and place it on a constant temperature magnetic stirrer at a constant temperature of 25°C, slowly Stir for 2 hours. During stirring, 2ml of 2M sodium hydroxide and 1ml of epichlorohydrin were added every 12 minutes for a total of 10 additions, accumulatively adding 20ml of 2M sodium hydroxide and 10ml of epichlorohydrin. Afterwards, the temperature was raised to 65° C., and constant temperature and slow stirring were continued for 2 hours. Transfer the reaction product to a sand core funnel, and use a vacuum pump to drain the activated Sepharose CL-4B. Then transfer Sepharose CL-4B to a beaker, add 50ml of 2M sodium carbonate, 5g of iminodiacetic acid, and 60mg of sodium borohydride, mix well, place on a constant temperature magnetic stirrer, keep the temperature at 60°C, and stir slowly for 15 hours, the react...
Embodiment 2
[0022] Take 40g of dried Sepharose 4 Fast Flow, put it into a 200ml beaker, add 20ml of 2M sodium hydroxide, 2ml of epichlorohydrin, and 80mg of sodium borohydride, mix well and place it on a constant temperature magnetic stirrer at a constant temperature of 30°C, slowly Stir for 3 hours. During stirring, 2ml of 2M sodium hydroxide and 1ml of epichlorohydrin were added every 12 minutes for a total of 10 additions, accumulatively adding 20ml of 2M sodium hydroxide and 10ml of epichlorohydrin. Afterwards, the temperature was raised to 65° C., and stirring was continued at a constant temperature for 3 hours. Transfer the reaction product to a sand core funnel, and use a vacuum pump to drain the activated Sepharose 4Fast Flow. Then transfer Sepharose 4 Fast Flow to a beaker, add 50ml of 2M sodium carbonate, 5g of iminodiacetic acid, and 70mg of sodium borohydride, mix well, place on a constant temperature magnetic stirrer, keep the temperature at 60°C, and stir slowly for 20 hour...
Embodiment 3
[0024] Take 5ml of Sepharose 4 Fast Flow-IDA, a prepared metal chelate chromatography filler, and transfer it to a chromatographic column with an inner diameter of 1.6cm. First wash off the residual ethanol in the filler with 10ml of phosphate buffer (pH7.4), and then sequentially Wash the column with 15ml, 200mM nickel sulfate, 10ml, 10% acetic acid, 50ml phosphate buffer (pH7.4). The lysate of Escherichia coli DH5α with the pET32a plasmid was loaded into the sample (both pET32a and DH5α can be purchased from Novagen), and after the crossing peak was exhausted, it was eluted with 200mM imidazole at a flow rate of 30 cm / hour, and the obtained The elution peak is pure thioredoxin with His-Tag. Another same volume of Escherichia coli DH5α expressing cell lysate with pET32a plasmid was taken, and the sample was loaded again, and after the crossing peak was exhausted, it was eluted with 200 mM imidazole at a flow rate of 150 cm / hour, and the obtained elution peak was with The thi...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap