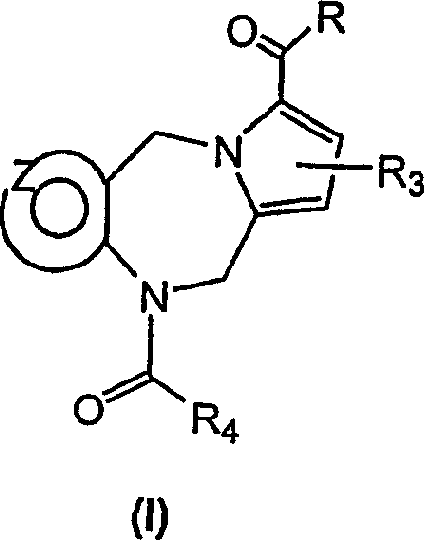
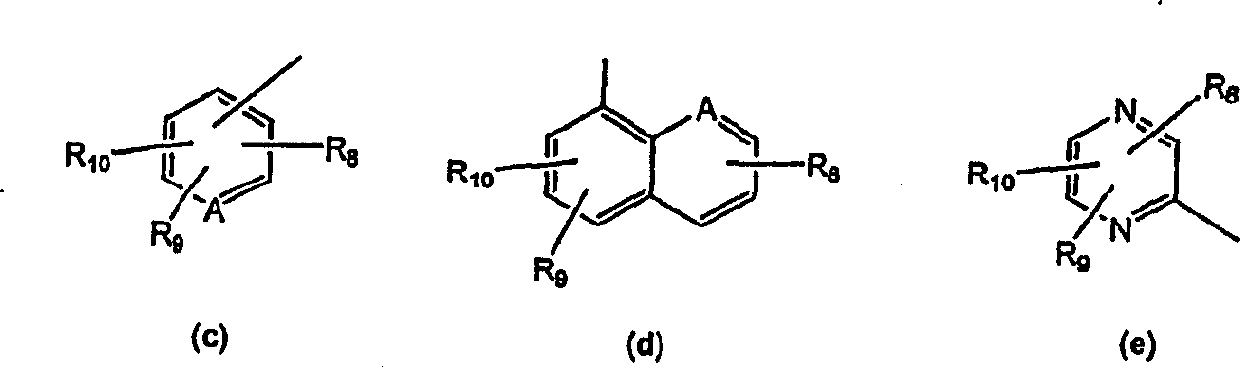
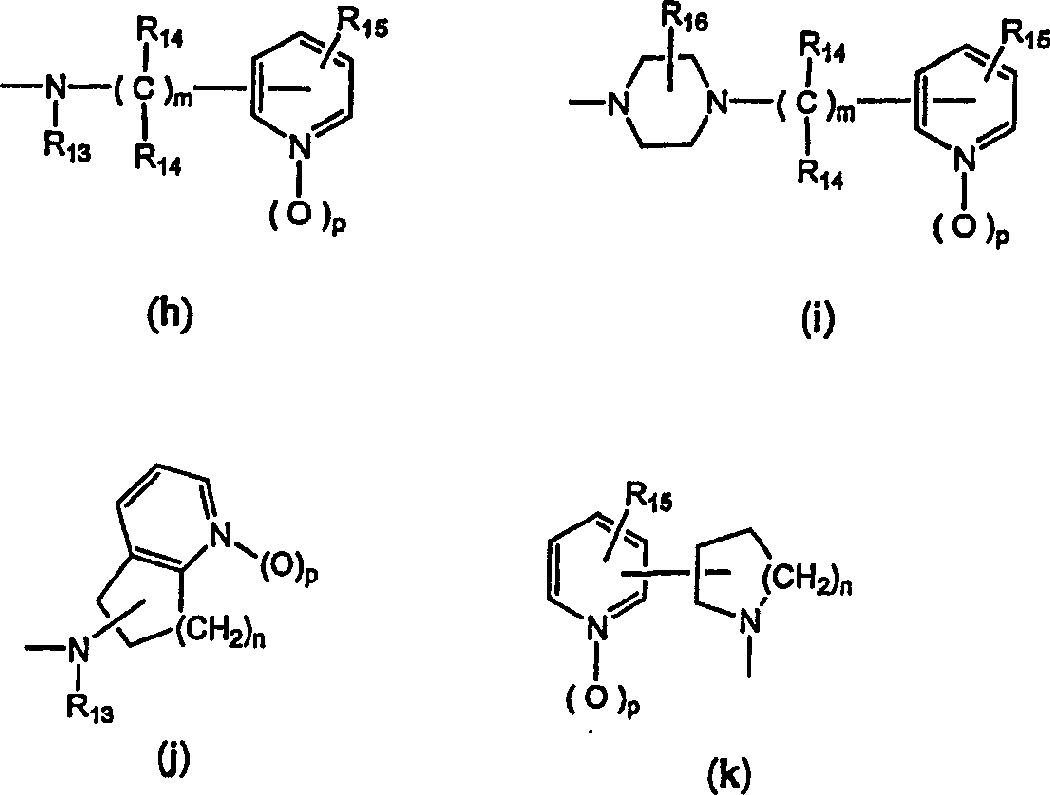
Novel tricyclic pyridyl benzazepine carboxyamides and derivatives their tocolytic oxytocin leceptor antagonists
A technology of alkyl and hydroxyl, applied in the field of novel tricyclic pyridyl carboxamide, which can solve the problem of lack of oral activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0086] The present invention also provides processes for the preparation of the compounds of the present invention.
[0087] The method of the invention
[0088] Compounds of the invention can be prepared according to one of the general methods outlined below.
[0089] The compound of general formula (I) can be prepared very conveniently by the method shown in scheme I, wherein R 4 is part B-C, wherein B is selected from (a) or (b), and C is selected from (c), (d), (e) and (f) as defined above.
[0090] Option I
[0091]
[0092] According to the preferred method above, in the presence of an organic base such as N,N-diisopropylethylamine (Hunig's base), in an aprotic organic solvent such as dichloromethane at a temperature ranging from -10°C to ambient temperature, The tricyclic diazepines of general formula (1), wherein R 3 and R 4 Reaction with a perhaloalkanoyl halide, preferably trichloroacetyl chloride, as defined above, affords the desired trichloroacetyl inter...
Embodiment 1
[0218] 10-[(2-Methyl-2'-trifluoromethyl-biphenyl-4-carbonyl)-10,11-dihydro-5H-pyrrolo[2,1-c][1,4]benzo Diazepin-3-yl]-(4-pyridin-4-yl-piperidin-1-yl)methanone
[0219] Step A. Methyl 4-bromo-3-methylbenzoate
[0220] To a suspension of 4-bromo-3-methylbenzoic acid (10.0 g, 46.5 mmol) in methanol (125 mL) was added concentrated sulfuric acid (1 mL). The reaction was heated overnight at reflux and a homogeneous solution was obtained after a few minutes of heating. After cooling, methanol was removed in vacuo and the residue was dissolved in dichloromethane, washed with saturated aqueous sodium bicarbonate. The organic phase was dried over anhydrous sodium sulfate, filtered and concentrated in vacuo to give the title compound as a brown solid, m.p. 41-43°C.
[0221] 1 H NMR (DMSO-d 6 , 400MHz): δ2.39(s, 3H), 3.85(s, 3H), 7.64-7.72(m, 2H), 7.88-7.89(m, 1H).
[0222] MS [El, m / z]: 228 [M]+.
[0223] C 9 h 9 BrO 2 Calculated values: C47.19, H3.90. Measured values: C47.22,...
Embodiment 2
[0254] Example 2: 10-{[2-methyl-2'-trifluoromethyl-[1,1'-biphenyl]-4-yl]carbonyl}-3-(4-[(1-pyridine oxide- 3-yl)methyl]piperazin-1-yl)carbonyl)-10,11-dihydro-5H-pyrrolo[2,1-c][1,4]benzodiazepine
[0255] Step A. 3-Chloromethyl-pyridine-1-oxide
[0256] To a solution of 3-hydroxymethyl-pyridine N-oxide (1.0 g, 8.0 mmol) in dichloromethane (40 mL) was added thionyl chloride (10 mL, 137 mmol). After stirring for 2 hours, the reaction mixture was concentrated in vacuo. The residue was partitioned between dichloromethane and saturated aqueous sodium bicarbonate. The aqueous layer was extracted repeatedly with dichloromethane. The combined organic layers were dried over anhydrous sodium sulfate, filtered and concentrated in vacuo to afford 0.60 g of the title product as a white solid, mp 133-137°C.
[0257] 1 HNMR (DMSO-d 6 , 400MHz): δ4.74(s, 2H), 7.40-7.45(m, 2H), 8.17-8.20(m, 1H), 8.35(s, 1H).
[0258] MS[(+)APCI, m / z]: 144[M+H]+.
[0259] C 6 h 6 ClNO analysis calculat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com