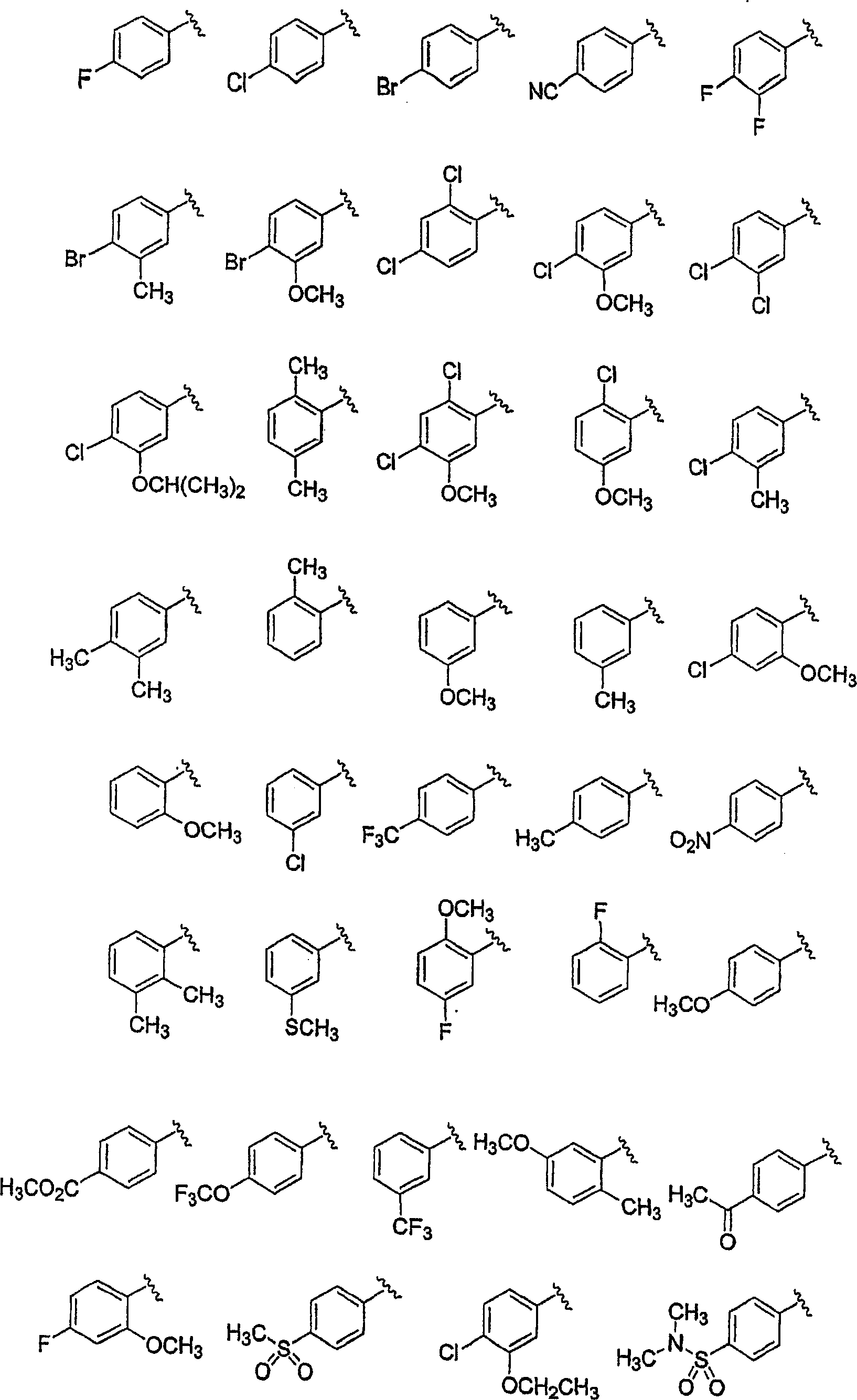
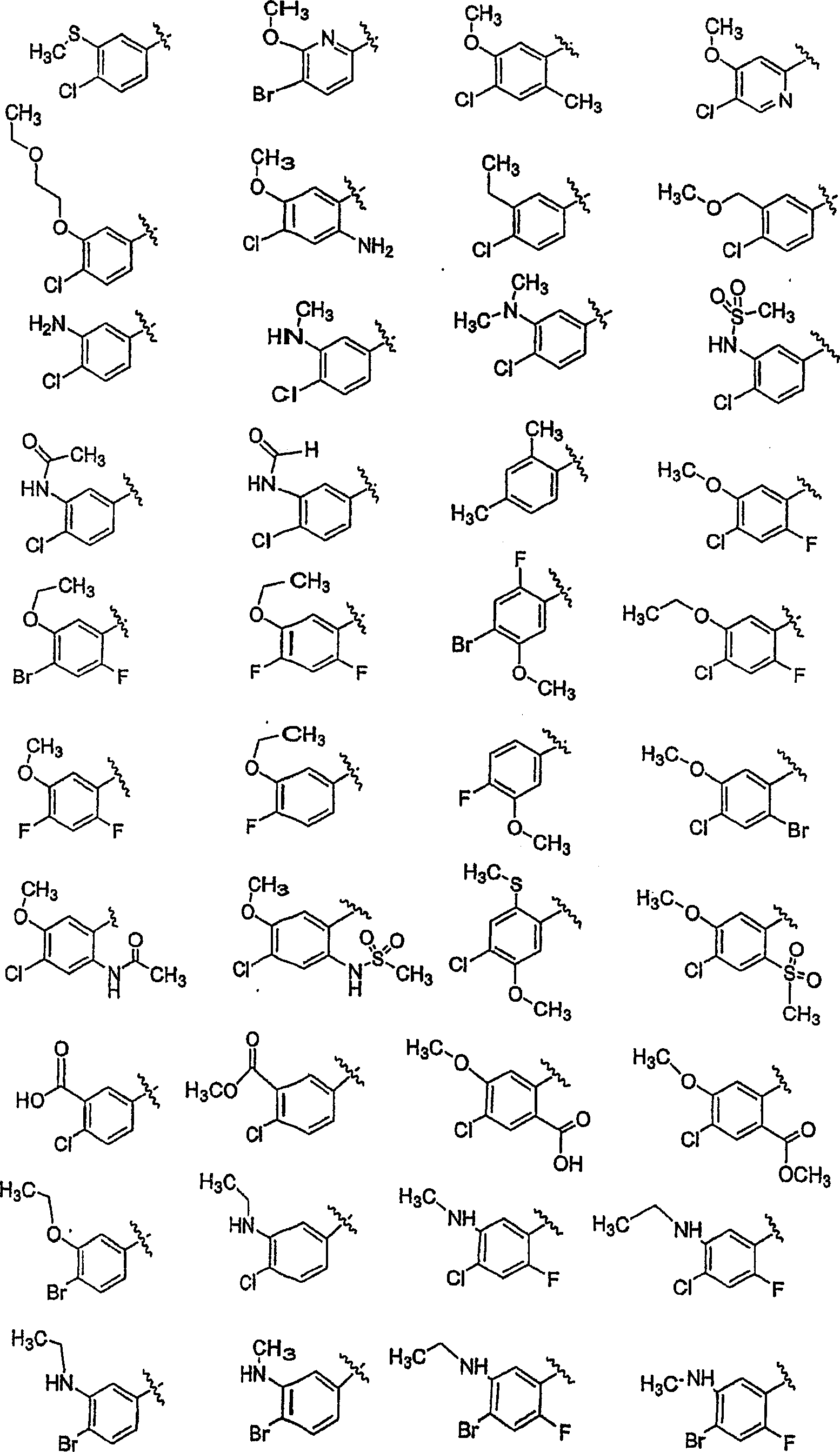
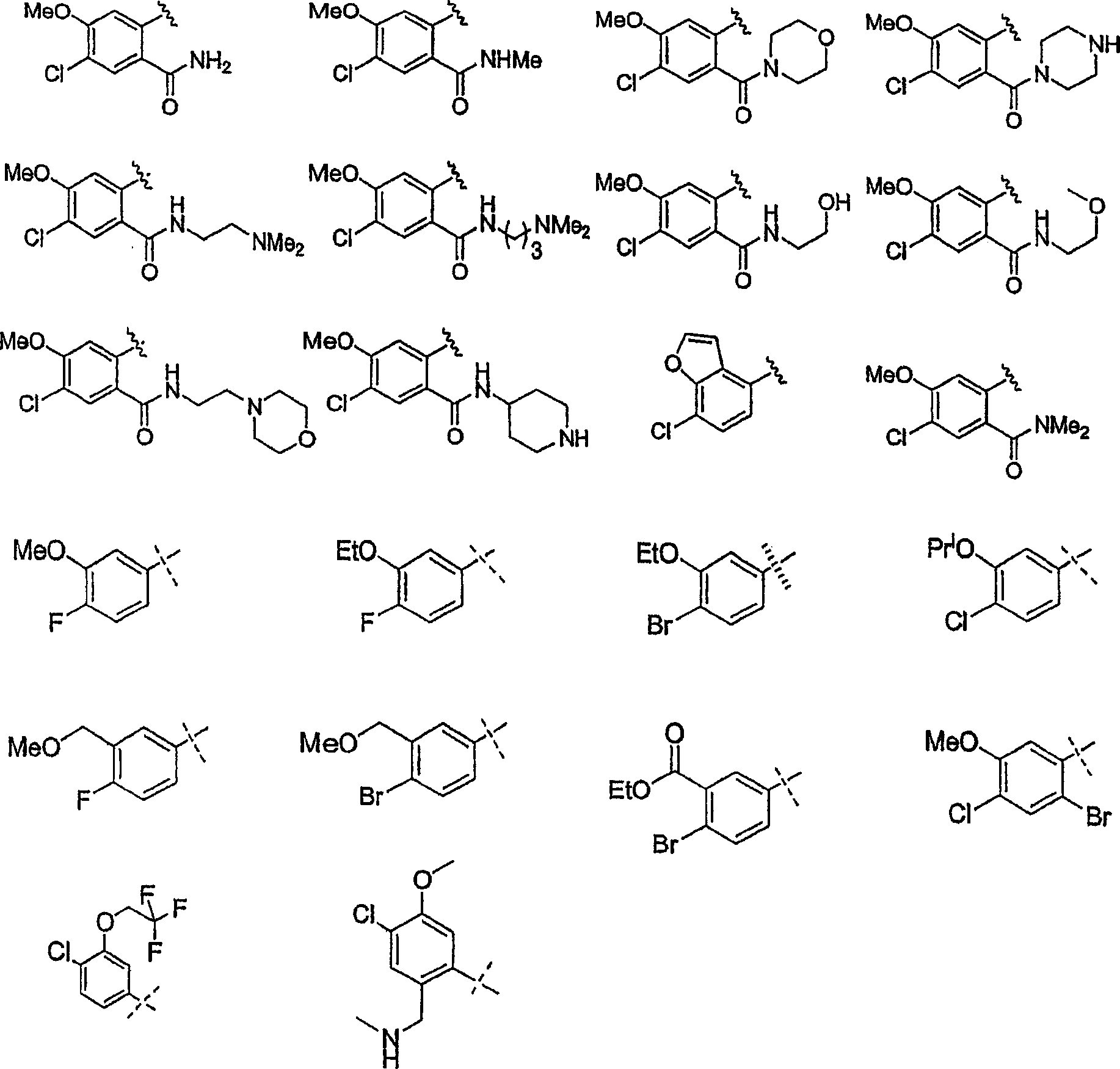
Substituted piperazines
A substituent and representative technology, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulations, organic chemistry, etc., can solve the problems of short half-life and the risk of degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0208] The piperazine ring can be formally attached to the terminal aryl unit in a variety of ways: through aromatic nucleophilic substitution reactions, metal-catalyzed coupling reactions (arylation of secondary amines), ring expansion, rearrangement, and cyclization reactions, among others. Different protection / deprotection strategies can also be employed. Thus, all or only a portion of the final molecular structure may be present at the critical aryl coupling step. Examples of various such aryl coupling methods are listed below.
[0209] Scheme A: Metal-catalyzed arylation of secondary amines
[0210] Synthesis of (5-chloro-2-piperazin-1-yl-phenyl)-phenyl-methanone
[0211]
[0212] At room temperature, piperazine (3.6g, 42.5mmol), palladium(II) acetate (0.007g, 0.043mmol), sodium tert-butoxide (0.22g, 2.4mmol) and BINAP (0.042g, 0.068mmol) were dissolved in 10 mL of anhydrous Stir in toluene for 15 minutes. Then, a solution of (2-bromo-5-chloro-phenyl)-phenyl-methan...
Embodiment 2
[1298] The protocol mentioned in the following examples is that described in Example 2.
[1299] Scheme A: Metal-catalyzed arylation of secondary amines
[1300] Synthesis of tert-butyl 1-[4-(4-chloro-3-methoxy-phenyl)-[1,4]diazepane-1-carboxylate:
[1301]
[1302] 5-Bromo-2-chloroanisole (1.10g, 5mmol, 1.0equiv), N-Boc-homopiperazine (1.0g, 1equiv), NaOtBu (0.72g, 1.5eq), rac-BINAP (58mg , 0.015 equiv) and Pd2Dba3 (28 mg, 0.005 eq) was heated in 3 mL of toluene at 90 °C overnight. After cooling to room temperature, the residue was dissolved in EtOAc and washed with water and brine. Na for organic layer 2 SO 4 Drying, filtration, evaporation, and flash column chromatography (1:4 EtOAc / hexanes) gave 1-[4-(4-chloro-3-methoxy-phenyl)-[1,4]diazepine tert-Butyl Hepane-1-carboxylate. 1 H NMR (400MHz, CDCl 3 )δ7.13(d, 1H), 6.22(d, 1H), 6.20(dd, 1H), 3.86(s, 3H), 3.45(m, 6H), 3.32(m, 2H), 3.20(m, 2H ), 1.95 (m, 2H), 1.20 (s, 9H). LCMS observed for (M+H-Boc)+: 241.
[130...
Embodiment 3
[2109] The preferred protocol in the following examples is that described in Example 3.
[2110] Scheme A: Metal-catalyzed arylation of secondary amines
[2111] Synthesis of 1-[4-(4-chloro-3-methoxy-phenyl)-piperazin-1-yl]-2-(4-chloro-5-methyl-3-piperazin-1-yl- Pyrazol-1-yl)-ethanone
[2112]
[2113] According to protocol A of Example 1, at 70 ° C, heat 2-(3-iodo-4-chloro-5-methyl-pyrazol-1-yl)-1-[4-(4- Chloro-3-methoxy-phenyl)-piperazin-1-yl]-ethanone (204mg, 1eq), piperazine (430mg, 10eq, 9,9-dimethyl-4,5-bis(benzene phosphino) xanthene (Xantphos) (40mg, 0.3eq), Pd 2 (dba) 3 (72mg, 0.1eq) and Cs 2 CO 3 (200mg, 1.5eq) mixture overnight, then cooled to room temperature, dissolved in EtOAc and washed with water. The organic layer was purified by reverse phase HPLC (acetonitrile-H with 0.1% TFA 2 O as eluent), resulting in the title compound: LCMS retention time: 3.07 min (Agilent Zorbax SB-C18, 2.1 x 50 mm, 5 μ, 35 °C) using a 4.5 min gradient from 20-95% B at 95% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com