Bromhexine Hydrochloride aseptic freeze-drying formulation for injection and its preparation method
A technology of bromhexine hydrochloride and freeze-dried preparation, applied in the field of sterile freeze-dried preparation of bromhexine hydrochloride for injection and preparation thereof, can solve the time and conditions of poor stability of dosage form, inconvenient for long-distance transportation, long-term preservation and storage Limitation and other issues to prevent degradation and improve bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
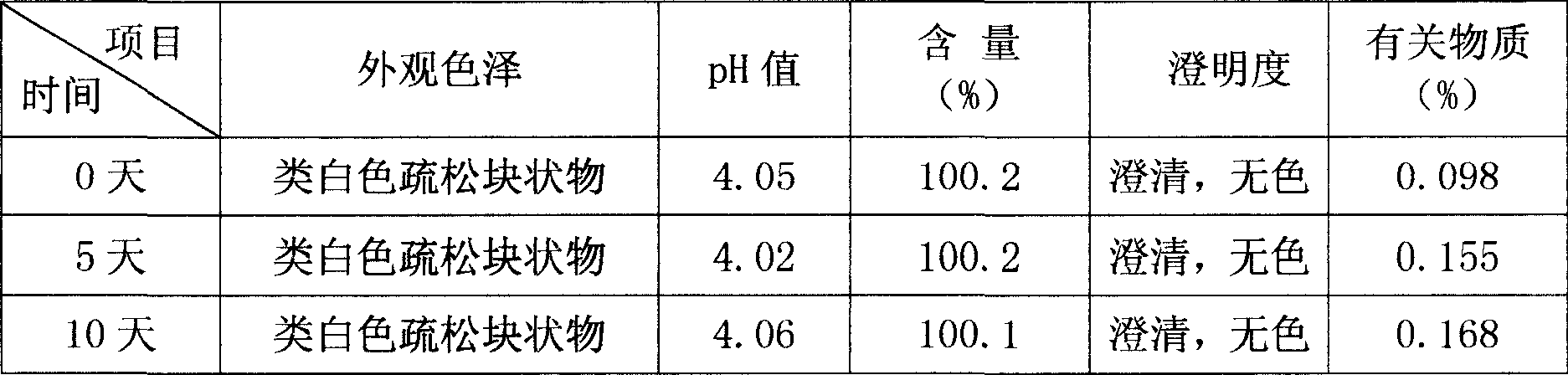
Image
Examples
Embodiment 1
[0026] Embodiment 1: the specification of 4mg / bottle. The formula of 1000 bottles of bromhexine hydrochloride sterile freeze-dried preparation for injection is:
[0027] 4g of bromhexine hydrochloride, 70g of mannitol, appropriate amount of citric acid, and water for injection were added to 2000ml, and a total of 1000 bottles were made.
[0028] Preparation:
[0029] 1) Weigh 70g of mannitol according to the prescription, add an appropriate amount of water for injection, stir to dissolve, add 0.2% (m / v) activated carbon for injection, stir well, keep warm in a hot water bath for 20-30 minutes, and then use a filter to Decarbonize by primary filtration, wash twice with a small amount of water for injection, and combine the filtrates to obtain the excipient formulation;
[0030] 2) Take 4 g of bromhexine hydrochloride, add about 3 / 4 of the amount of water for injection to dissolve it, adjust the pH value to 2.0-3.5 with 0.1 mol / L phosphoric acid, and obtain bromhexine hydrochl...
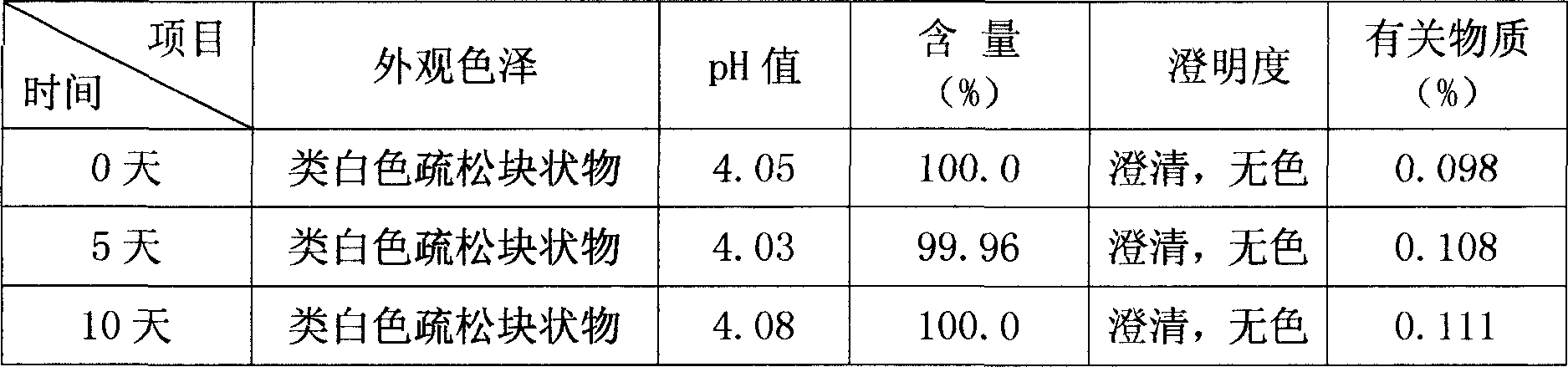
Embodiment 2
[0037] Embodiment 2: 4mg / bottle specification. The formula of 1000 bottles of bromhexine hydrochloride sterile freeze-dried preparation for injection is:
[0038] 4.0g of bromhexine hydrochloride, 70g of glucose, 100g of mannitol, appropriate amount of phosphoric acid, and water for injection were added to 2000ml, and a total of 1000 bottles were prepared.
[0039] Preparation:
[0040] 1) Weigh 70g of glucose and 100g of mannitol according to the prescription, add an appropriate amount of water for injection, stir to dissolve, add 0.2% (m / v) activated carbon for injection, stir evenly, keep warm in a hot water bath for 20-30 minutes, and use The filter is pre-filtered and decarbonized, washed twice with a small amount of water for injection, and the filtrate is combined to obtain the excipient formulation;
[0041] 2) Take 4.0 g of bromhexine hydrochloride according to the prescription, add about 3 / 4 of the amount of water for injection to dissolve it, adjust the pH value t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com