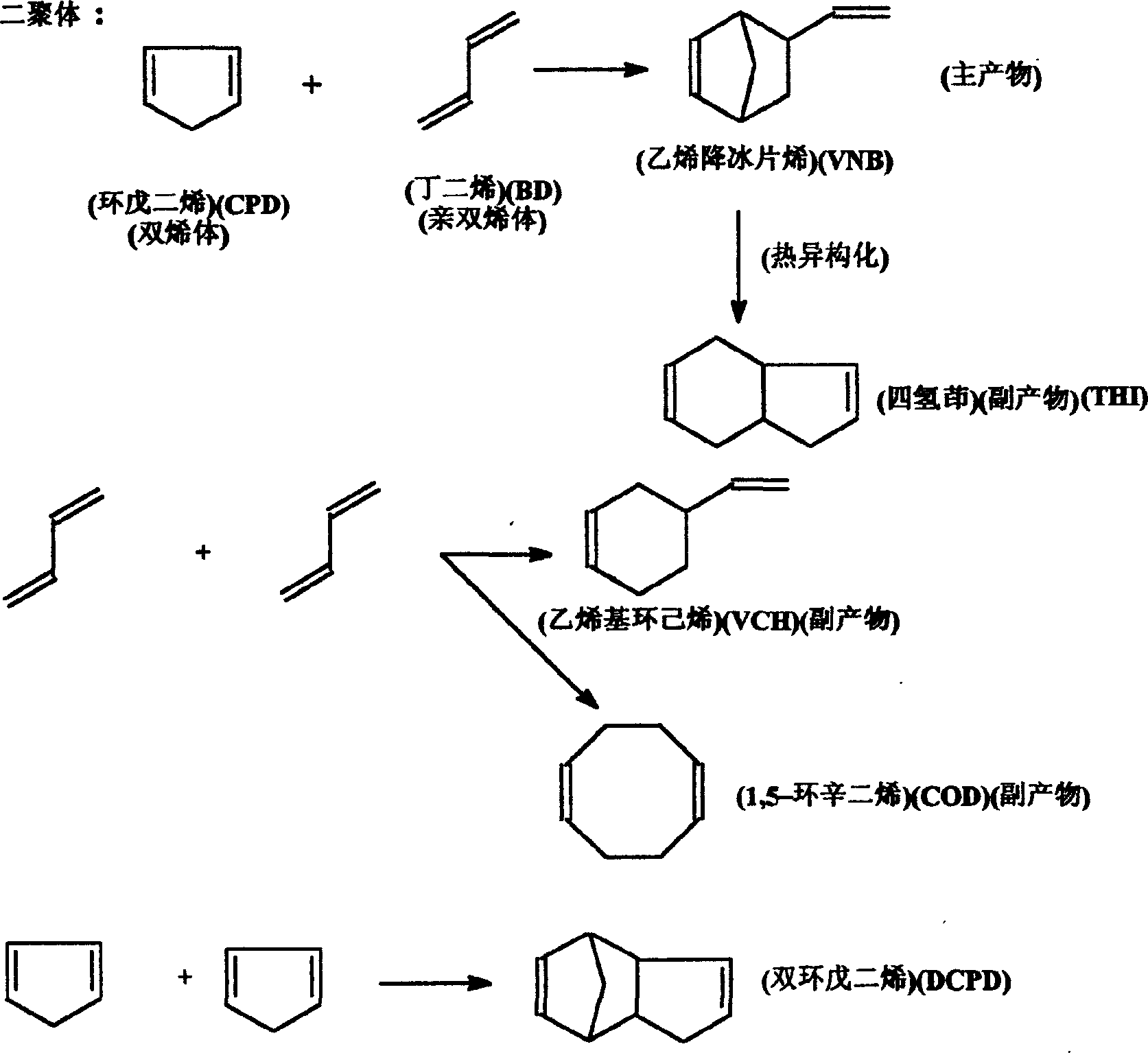
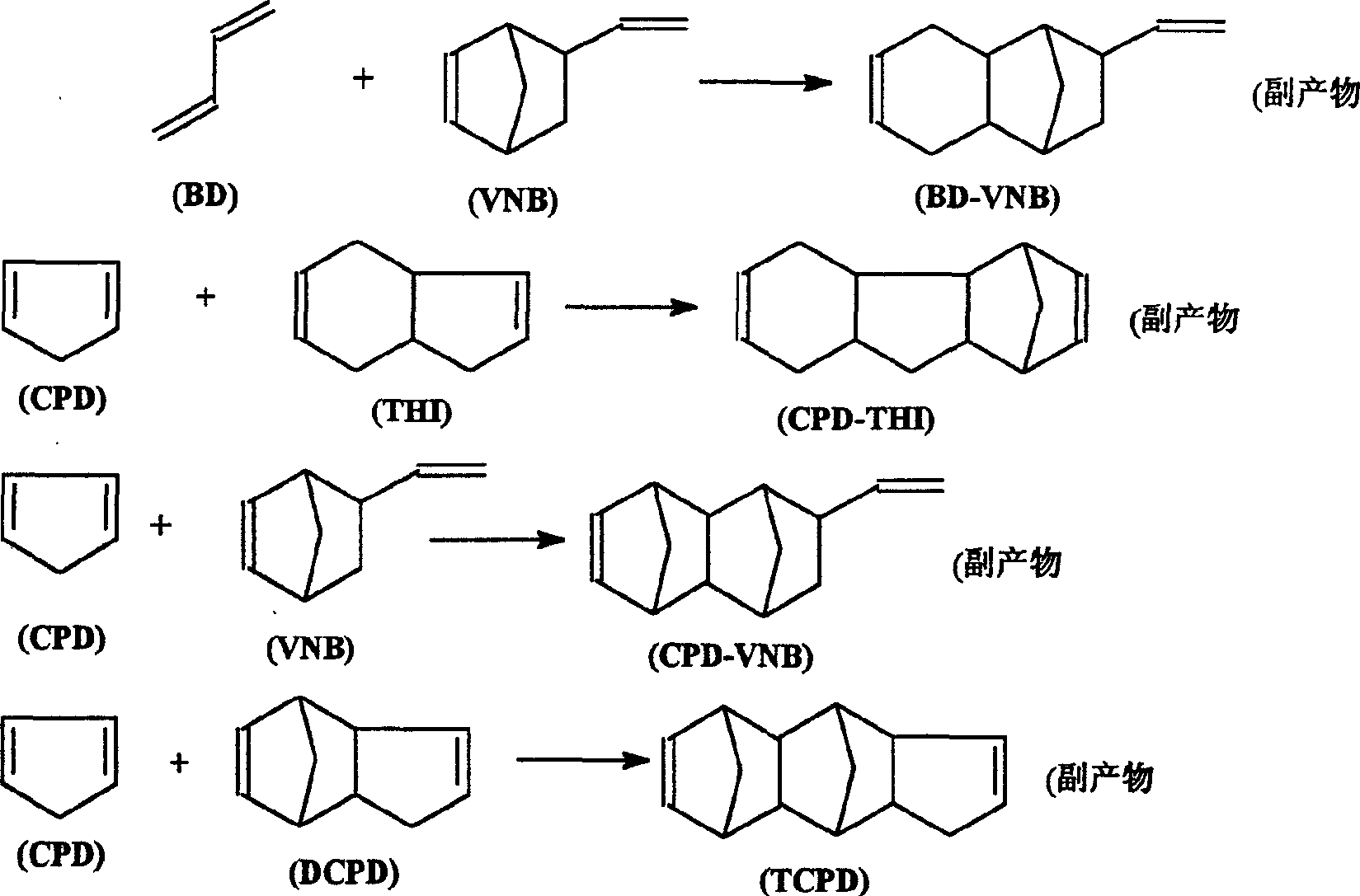
Method for synthesizing 5-vinyl-2-norbornaene
A synthesis method and a technology for norbornene, which are applied in the field of synthesis of 5-vinyl-2-norbornene, can solve the problems of low selectivity and yield of main products, and achieve strong adaptability, flexible operation, and the realization of tube effect of reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 325g BD (purity: 98.636wt%), CPD 310.0g (containing 93.26% CPD, 6.276% DCPD), (BD: CPD = 1.27: 1mol ratio = 1.039: 1wt ratio), 225g toluene and 0.5g (about 600ppm) α -Naphthol was added into a 1L autoclave, stirred at a speed of 460 rpm and gradually heated to 154-155°C, and reacted for 3 hours at 2.1Mpa. After the reaction was completed, the mixture was cooled with cooling water to obtain 825 g of the reaction solution. Cool the reactor bladder to -20°C in a freezer. Sampling is carried out chromatographic analysis, and its result is as follows:
[0035] The product composition is: BD: 5.511wt% = 44.64g; CPD: 6.178wt% = 50.05g; VCH: 9.32wt% = 75.49g; VNB: 17.669wt% = 143.12g; Toluene: 24.083wt% = 195.07g; COD : 0.466wt% = 3.775g; THI: 5.751wt% = 46.58g; DCPD: 30.085wt% = 243.69g.
[0036] CPD conversion = 93.7wt% = 93.31mol%
[0037] VNB selectivity = 60.19wt% = 33.10mol%
[0038] VNB yield = 50.40wt% = 30.89mol%
Embodiment 2
[0040] 300g BD (purity: 98.636wt%), CPD 315.0g (containing 90.2% CPD, 8.0% DCPD), (BD: CPD = 1.081: 1mol ratio = 0.956: 1wt ratio), 225g toluene and 0.5g (about 600ppm) α -Naphthol was added to a 1L autoclave, stirred for 5 minutes before the reaction, then stopped stirring, heated up to 154-155°C, and reacted at 2.1Mpa for 3 hours. After the reaction was completed, cooled with cooling water to obtain 825g of the reaction solution. Put the reactor bladder into the freezer to cool to -20°C. Sampling and chromatographic analysis, the results are as follows:
[0041]The product composition is: BD: 5.511%=44.64g; CPD: 6.178wt%=50.04g; VCH: 9.32wt%=75.49g; VNB: 17.669wt%=143.12g; Toluene: 24.083wt%=195.07g; COD: 0.466 wt% = 3.775g; THI: 5.751 wt% = 46.58g; DCPD: 35.085 wt% = 243.69g.
[0042] CPD conversion rate = 82.84wt% = 82.80mol%
[0043] VNB selectivity = 59.22wt% = 32.23mol%
[0044] VNB yield = 49.03wt% = 26.90mol%
Embodiment 3
[0046] 300g BD (purity: 98.636wt%), CPD 315.0g (containing 92.6% CPD, 4.6% DCPD), (BD: CPD = 1.27: 1mol ratio = 1.039: 1wt ratio), 225g toluene and 0.5g (about 600ppm) α -Naphthol was added to a 1L autoclave, stirred for 5 minutes before the reaction, then stopped stirring, and gradually heated up to 154-155°C, and reacted for 1 hour at 2.1Mpa. After the reaction was completed, the mixture was cooled with cooling water to obtain 825 g of the reaction solution. Cool the reactor bladder to -20°C in a freezer. Sampling is carried out chromatographic analysis, and its result is as follows:
[0047] The product composition is: BD: 9.429%=12.75g; CPD: 6.406wt%=34.3g; VCH: 5.845wt%=43.39g; VNB: 14.984wt%=126.6g; Toluene: 24.083wt%=195.07g; COD: 0.466wt% = 3.775g; THI: 2.688wt% = 22.71g; DCPD: 35.063wt% = 296.28g.
[0048] CPD conversion rate = 87.92wt% = 87.90mol%
[0049] VNB selectivity = 50.7wt% = 27.91mol%
[0050] VNB yield=44.57wt%=mol%=24.53mol%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com