Process for the preparation of benazepril
A molecular formula, the technology of ethyl phenylbutyrate, which is applied in the application field of trifluoromethanesulfonate to prepare the angiotensin-converting enzyme inhibitor benazepril, can solve the problem that the commercial scale has defects and cannot be completely unsatisfactory. Satisfaction, low total yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
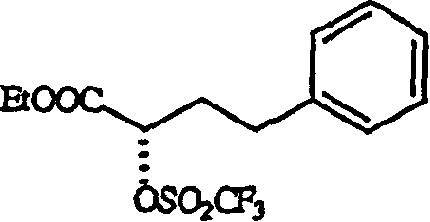
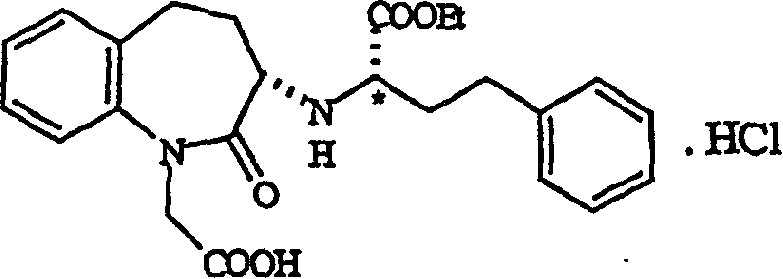
[0031] Preparation of hydrochloric acid (3S)-1-(carboxymethyl-[[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]2,3,4,5-tetrahydro-1H-[ 1] Benzazepin-2-one
[0032] 5.67 g of ethyl (R)-2-hydroxy-4-phenylbutyrate (99% ee), 2.79 g of pyridine were added to dichloromethane and cooled to -20°C. 10 g of trifluoromethanesulfonic anhydride dissolved in dichloromethane was added over a period of 15 to 20 minutes. Then, the reaction mixture was stirred at -20°C-25°C for 30 minutes and monitored by TLC. After the reaction was completed, the mixture was directly passed through a silica gel column (25 g, 60-125 mesh, 1 inch in diameter) with dichloromethane as the eluent. The fractions were combined and the solvent was removed to give ethyl (R)-2-(trifluoromethanesulfonyloxy)-4-phenylbutanoate (ie, triflate) as an oil. At 30 to 35°C, the oil was dissolved in 15 ml of dichloromethane, and added dropwise to 5.67 g of 1-tert-butoxycarbonylmethyl-3-S-amino-2,3,4,5-tetrahydro- In a mixture fo...
Embodiment 2
[0035] Preparation of hydrochloric acid (3S)-1-(carboxymethyl-[[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]-2,3,4,5-tetrahydro-1H- [1] Benzazepin-2-one
[0036] (R)-2-(Trifluoromethanesulfonyloxy)-4-phenylbutanoic acid ethyl ester (ie, trifluoromethanesulfonate) was prepared by the method described in Example 1 to obtain an oil. The oil was dissolved in 15ml of dichloromethane, and 5.67 grams of 1-tert-butoxycarbonylmethyl-3-S-amino-2,3,4,5-tetrahydro-1H-(1)- A solution formed by dissolving benzazepin-2-one and 2.46 g of N-methylmorpholine in dichloromethane. The reaction mixture was stirred for 1 hour and subjected to post-treatment similar to Example 1 to obtain benazepril hydrochloride, 8.20 g, almost white powder, the diastereomer ratio SS:SR=99.35:0.15, yield 91.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com