Aldhyde used as component for perfuming or flavouring
A technology of components and flavoring agents, applied in the directions of essential oils/spice, detergent composition spices, food science, etc., can solve problems such as lack of performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
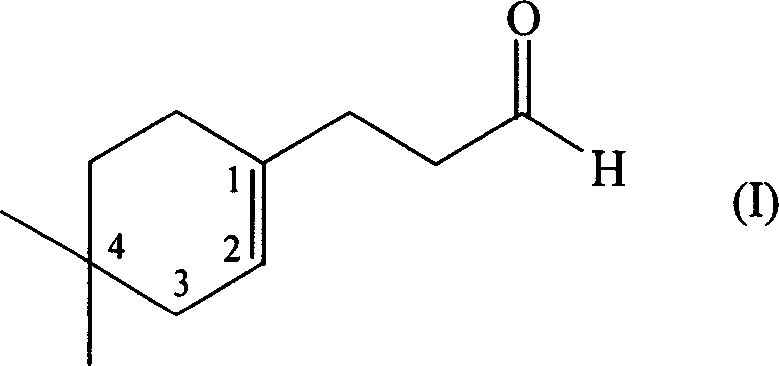
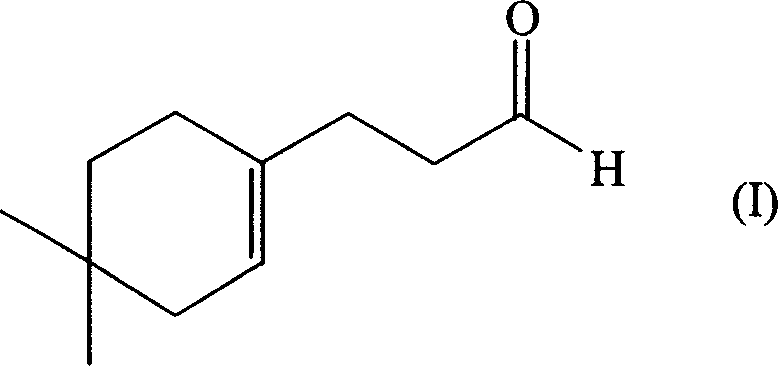
[0047] Synthesis of 3-(4,4-Dimethyl-1-cyclohexen-1-yl)propanal
[0048] a) Synthesis of 3-(4,4-dimethyl-cyclohex-1-enyl) butyl propionate
[0049] Throw in 4,4-dimethyl-cyclohexanol (100g, 0.78mol) in the 500ml three-necked round bottom flask, heat to 160 ℃, add tBuOOtBu (11.4g, 0.08mol) and ethacrylic acid over 4 hours at the same time Ester (39.2 g, 0.39 mol). Ten minutes after the addition was complete, the mixture was cooled to 50°C and the lights were distilled off. A solution of 30% w / w NaOH (61 g) and MTBE (200 g) in water was then added. Stirring was continued overnight at 40°C and the aqueous phase was separated. The aqueous phase was back extracted 4 times with MTBE (200 g). MTBE (200 g) was then added to the aqueous phase and washed with 50% w / w aqueous H 2 SO 4 Adjust the pH to 1. The organic phase was washed with water and concentrated to afford 49.4 g of crude 8,8-dimethyl-1-oxa-spiro[4.5]decan-2-one (GC purity: 96.3%).
[0050] Crude 8,8-dimethyl-1-oxa...
Embodiment 2
[0067] Preparation of Perfuming Compositions
[0068] A perfuming composition with a verbena scent is prepared by mixing the following components:
[0069] Component parts by weight
[0070] Benzyl acetate 100
[0071] 50% *Benjoin Sumatra Essential Oil 80
[0072] Bergamot Essential Oil 150
[0073] Citral 50
[0074] Coumarin 50
[0075] Geraniol 350
[0076] 1,3-Benzodioxole-5-carbonaldehyde 50
[0077] Lemongrass 100
[0078] Muscenone 1) 20
[0079] 950
[0080] * in dipropylene glycol
[0081] 1) Methyl-cyclopentadecenone, place of origin: Firmenich SA, Geneva, Switzerland
[0082] Add 50 parts by weight of 3-(4,4-dimethyl-1-cyclohexen-1-yl) propionaldehyde in the above-mentioned perfuming composition, which endows the composition with a natural floral fragrance type, which not only improves the fragrance The amount increases the sensory diffusion. Also, the citrus / citral notes of the composition are more pleasantl...
Embodiment 3
[0085] Preparation of Perfuming Compositions
[0086] Perfuming compositions of the linden type are prepared by mixing the following components:
[0087] Component parts by weight
[0088] Benzyl acetate 20
[0089] Lauryl acetate 70
[0090] 2-Phenyl-1-propanol 190
[0091] Anisaldehyde 10
[0092] 10% * Cuminaldehyde 220
[0093] Cassis Base 345B 1) 120
[0094] cis-3-Henenol 5
[0095] 10% * Delphone 2) 20
[0096] 1,3-Benzodioxole-5-carbonaldehyde 3) 20
[0097] Alpha Ionone 20
[0098] Mayol 4) 240
[0099] 1-(4-Methylphenyl)-1-ethanone 5
[0100] 10% * Neobutenone 5) 10
[0101] 10% * Methyl 2-nonynoate 60
[0102] 10% * (2E,6Z)-2,6-Nadienal 10
[0103] Phenyl ethyl alcohol 20
[0104] Methyl salicylate 5
[0105] Terpineol 20
[0106] Veloutone 6) 5
[0107] Violettyne MIP 7) 30
[0108] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com