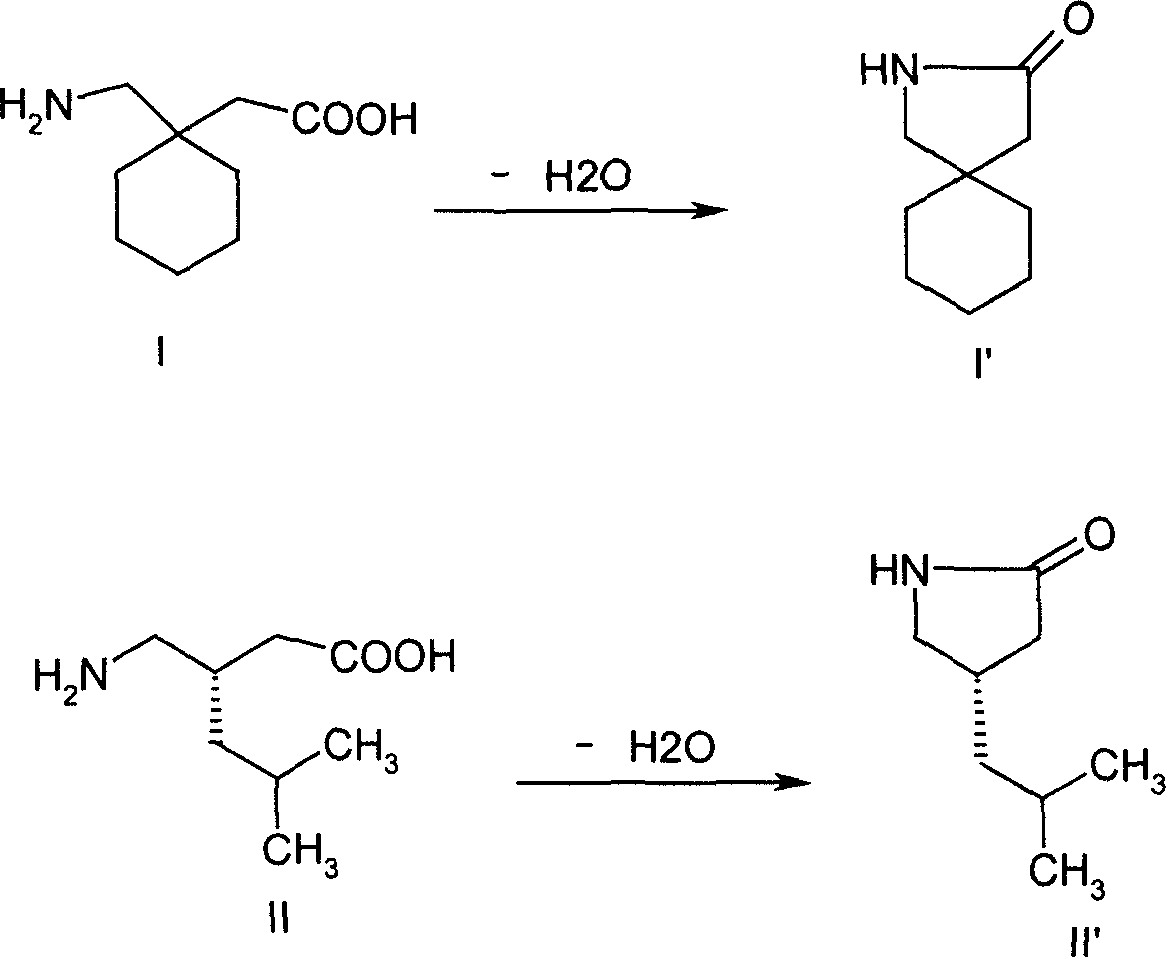
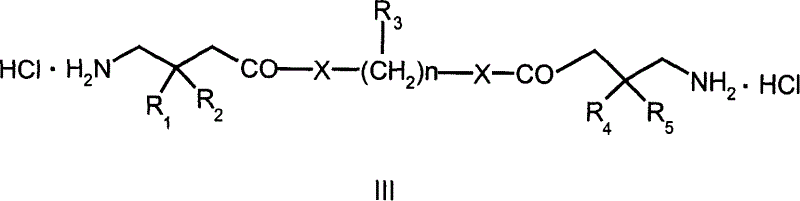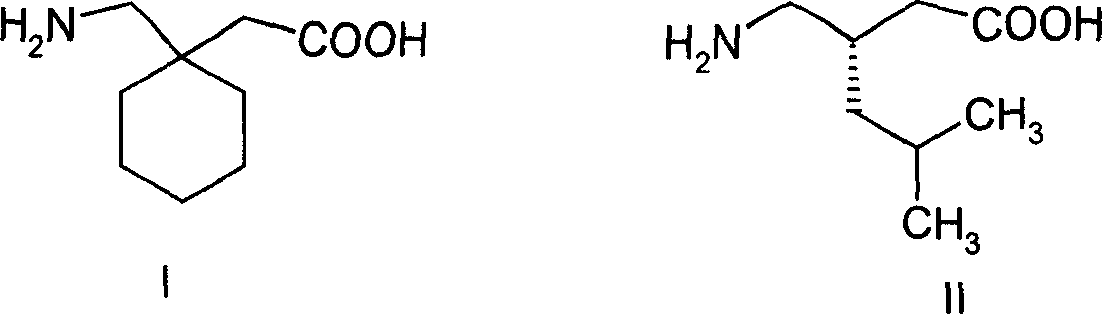Interconnected medicine precursor of gabapentin and pregabalin and its medicinal use
A technology of pregabalin and interconnection, which is applied in the interconnection prodrug of gabapentin and pregabalin and the field of its medical application, can solve the problems of accelerated lactam by-products, uncontrollable drug quality, etc., and achieves good antiepileptic, Good for the treatment of neuropathic pain and prolongation of biological half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Gabapentin (IV) protected by tert-butoxycarbonyl (BOC) 1 )Synthesis
[0031] Add 0.80g (0.02mol) of NaOH to a 250ml eggplant-shaped bottle, add 20ml of water to dissolve, ice bath, and electromagnetically stir, then add 3.42g (0.02mol) of gabapentin, after the solid is completely dissolved, alternately add 5.24g (0.024mol) of BOC anhydride mol) dissolved in 30ml of dioxane and 0.88g of NaOH dissolved in 30ml of water, keeping the pH value of the reaction solution at about 9, the addition was completed in about 30 minutes. After the addition, the mixture was reacted in ice bath for 2 hours, and then at room temperature for 2 hours. The reaction is complete. Extract the excess BOC anhydride in the reaction solution with ether, 30ml x 5 times in total, discard the ether layer, add 5% citric acid to the water layer under stirring to about pH 3, solids are precipitated, add 80ml ethyl acetate to extract the precipitate, and add 80ml of ethyl acetate to extract th...
Embodiment 2
[0032] Example 2 Pregabalin (IV) protected by tert-butoxycarbonyl (BOC) 2 )Synthesis
[0033] With reference to the method of Example 1, taking pregabalin as raw material and BOC anhydride reaction, obtain the III of white solid 2 , yield 90.73%, mp: 70~71℃; MS (ESI, m / e): 258.4(M-1), 260.3(M+1), 282.3(M+Na);
[0034] TLC: developing solvent petroleum ether: EtAc: CH 3 OH (5:2:0.5), Rf = 0.34.
Embodiment 3
[0035] Synthesis of the gabapentin glycol monoester of embodiment 3 tert-butoxycarbonyl protection (V 1 )Synthesis
[0036] Add IV to 100ml eggplant-shaped bottle 1 2.5g (0.00922mol), anhydrous CH 2 Cl 2 20ml, ice bath, electromagnetic stirring, to dissolve the solid, and then add ethylene glycol 3.43g (0.00922mol × 6) dissolved in 5ml of anhydrous DMF solution. After cooling to 0°C, DCC1.99g (0.00922mol×1.05) was dissolved in 5ml anhydrous CH 2 Cl 2 After reacting in an ice bath for 1 hour, 0.25 g of DMAP was added, then the ice bath was removed, and the reaction was carried out at room temperature for 12 hours. After the reaction was completed, the precipitated DCU was filtered off, the solvent was evaporated under reduced pressure, dissolved in 100 ml of EtAc, and the precipitated DCU was filtered again, and the EtAc layer was washed with 5% NaHCO 3 Wash 30ml×3 times, wash 25ml×2 times with 5% citric acid, wash with 5% NaHCO 3 Wash 30ml x 3 times, wash 30ml x 3 time...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com