Inspection for quality control of Longxuejie
A technology for control and detection, dried dragon's blood, which is applied in the field of detection of Chinese herbal medicines and can solve problems such as difficulty in accurately reflecting product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
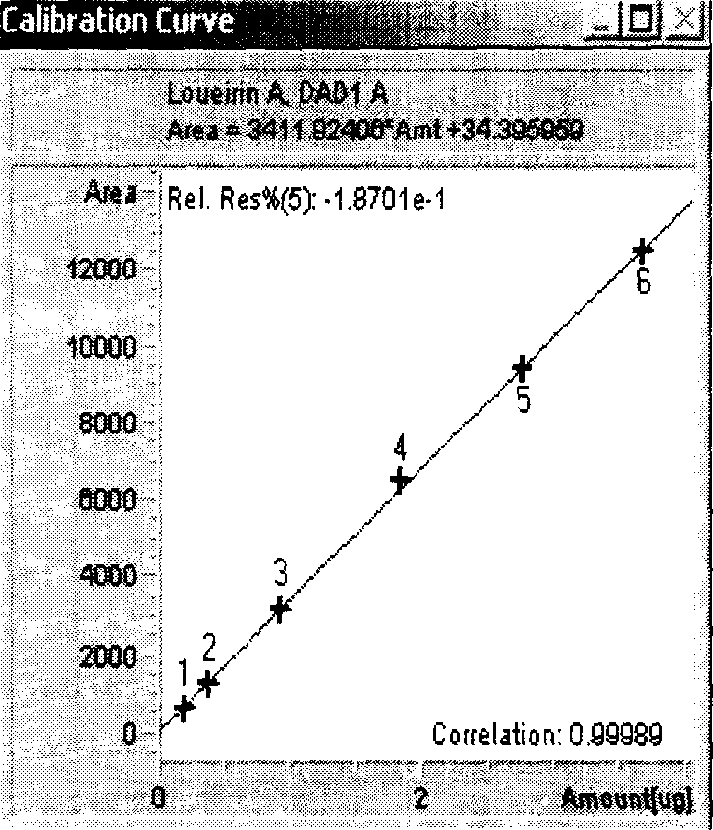
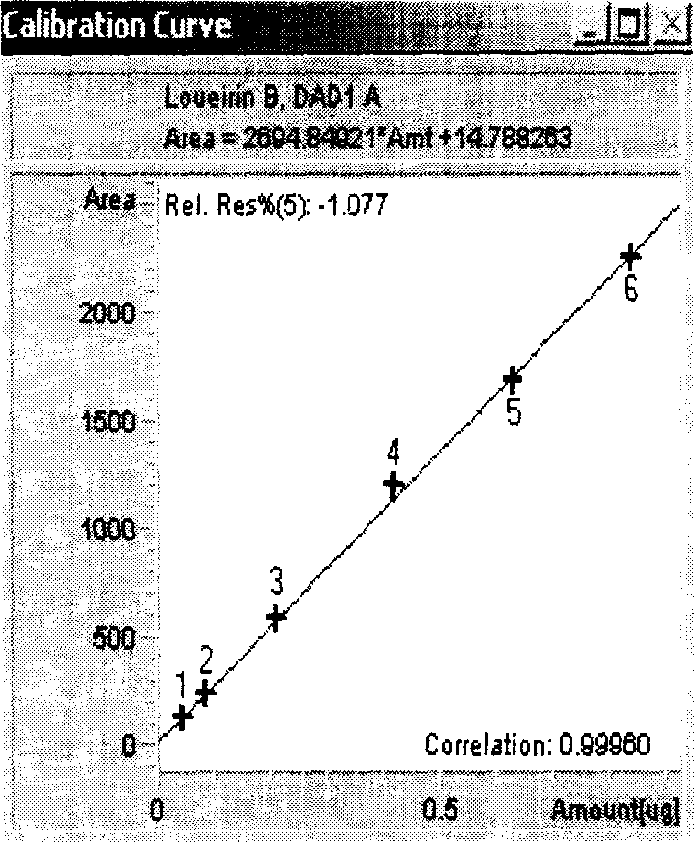
[0092] Chromatographic conditions and system suitability test Octadecylsilane bonded silica gel was used as filler; glacial acetic acid solution (1→100)-acetonitrile (63:37) was used as mobile phase, and the detection wavelength was 275nm. Column temperature: 30°C. The number of theoretical plates should not be less than 5,000 based on the calculation of the peaks A and B of dragon blood, and the separation degree between peak A and peak B of dragon blood should be greater than 2.0.
[0093] Preparation of Reference Substance Solution Accurately weigh the appropriate amount of reference substances of dragon blood A and dragon blood B which have been dried under reduced pressure by phosphorus pentoxide for 24 hours, add methanol to dissolve and make each 1ml containing dragon blood A 0.18mg, containing dragon blood A mixed solution of 0.04 mg of element B is obtained.
[0094] Preparation of the test solution Take about 0.25g of the powder of this product (passed through a No....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com