Method for purification of milbemycins
A kind of technology of acaricid and refining method, applied in the direction of organic chemistry, etc., can solve the problems of low productivity, low carrier load, high cost of refined products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
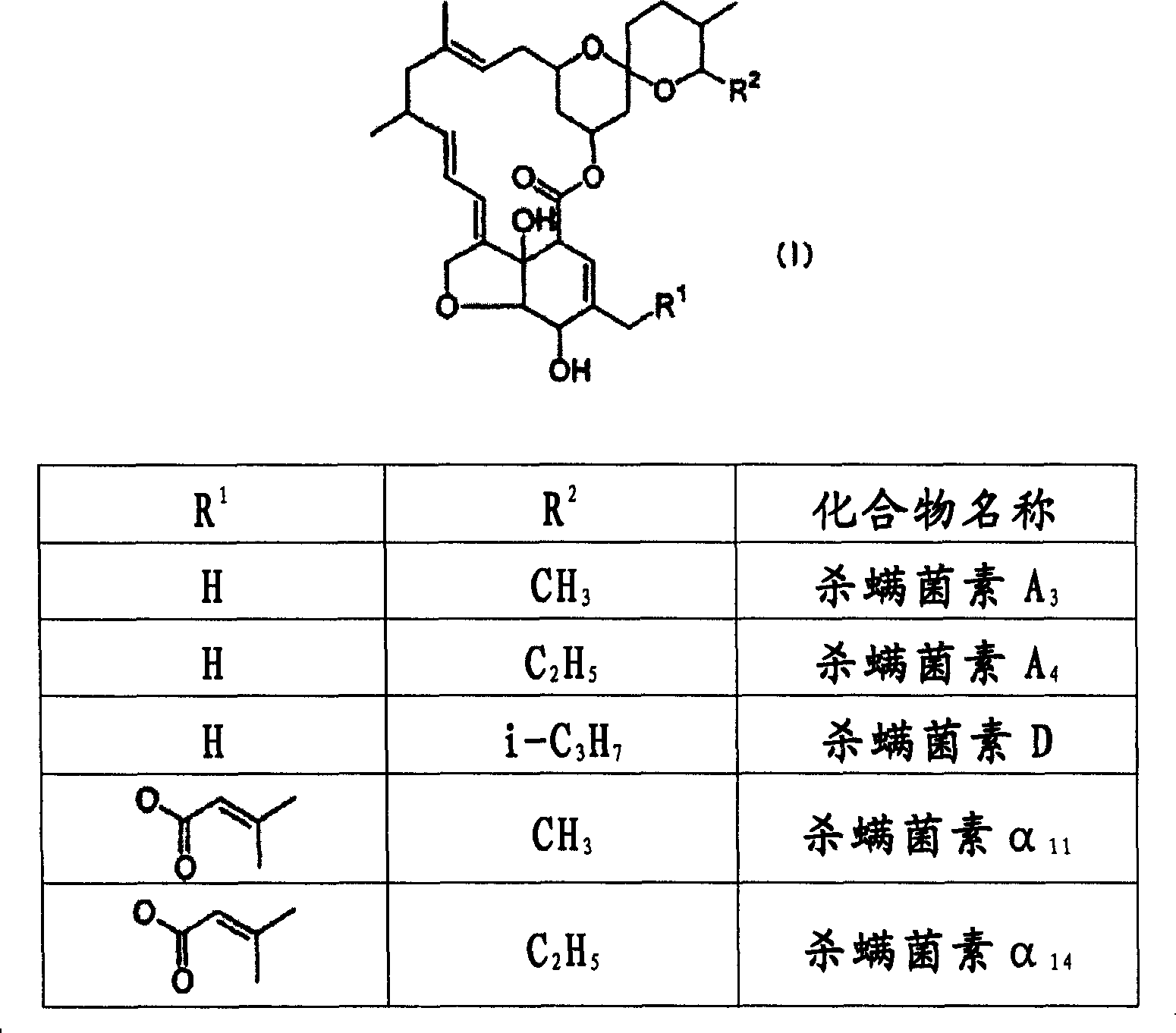
[0043] Mitetoxin A 3 and acaricidin A 4 Ethyl acetate solution was prepared from the residue of the fermentation broth. As a result of concentrating a part under reduced pressure, it was found that the concentration residue had a purity of 28% (Acaricidin A 3 : 4.2%; Acaricidin A 4 : 23.8%). This ethyl acetate solution (130 ml) was washed successively with 63 ml of 5% aqueous ammonia and 39 ml of 5% aqueous sulfuric acid. To the residue obtained by concentrating the ethyl acetate solution, 300 ml of methanol and 300 ml of water were sequentially added. Then, extraction was carried out with ISOPAR-E (hydrocarbon solvent manufactured by Exxonmobil: octane: 60-70%, nonane: 30-40%). The resulting ISOPAR-E solution was concentrated under reduced pressure, recrystallized, and then filtered to obtain acaricidin A 3 and acaricidin A 4 3.06 g of crystals (yield 75%). Its purity is 95% (Acaricidin A 3 : 15.2%; Acaricidin A 4 : 79.8%).
[0044] Acaricidin A 3 and acaricidin A...
reference example 1
[0051] Concentrated acaridin A 3 and acaricidin A 4 1 L of methanol solution prepared from the residue of the fermentation culture broth, and the obtained residue was adsorbed to silica gel (Using Wakol C-100, 400 g) column chromatography. It was developed in ethyl acetate-hexane mixed solution, and the concentrated product contained acaricidin A 3 and acaricidin A 4 fractions. After the obtained residue was recrystallized, 2.69 g of crystals of acaricidin were obtained by filtration (yield 78%). Its purity is 95% (Acaricidin A 3 : 14.3%; Acaricidin A 4 : 80.7%).
[0052] The present invention provides a method for purifying acaricids that does not use chromatography, is simple, economical, and has a high purification effect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com