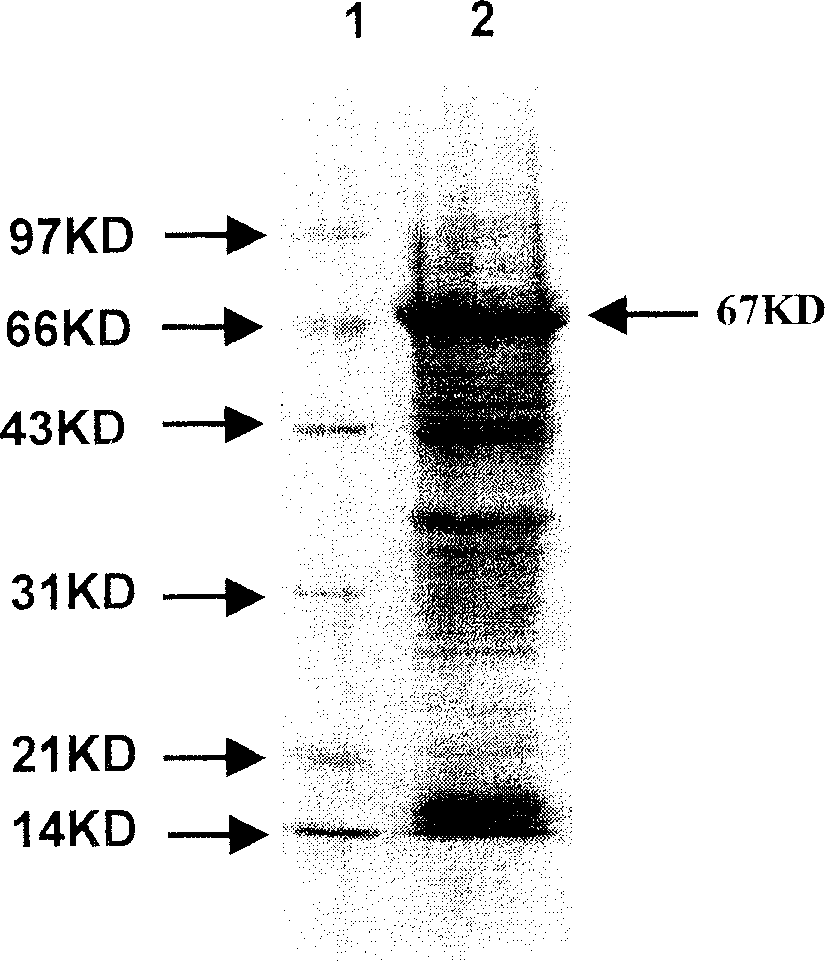
Heat shock protein 65- multiple epitope hepatitis B virus core antigen recombinant protein ú¿HSP65-HBcAgú®
A technology of hepatitis B virus and heat shock protein, applied in the field of genetic engineering recombinant fusion protein, can solve the problem of ineffective activation of CTL
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1 Obtaining the Encoding Gene of 65KD Heat Shock Protein (HSP65)
[0064]1. BCG: from Changchun Institute of Biological Products. The Sutong potato medium is used to cultivate BCG at a temperature of 37-39°C, and the grown BCG presents dry wrinkled light yellow pellicle. The pellicles were collected from which BCG genomic DNA was extracted.
[0065] 2. Extraction of BCG genomic DNA: the method refers to the book Molecular Cloning (J.Sambrook, Isolation of high-molecular-weight DNA from mammalian cells, 9.16-9.22, ColdSpring Harbor Laboratory Press, Molecular cloning, 1989).
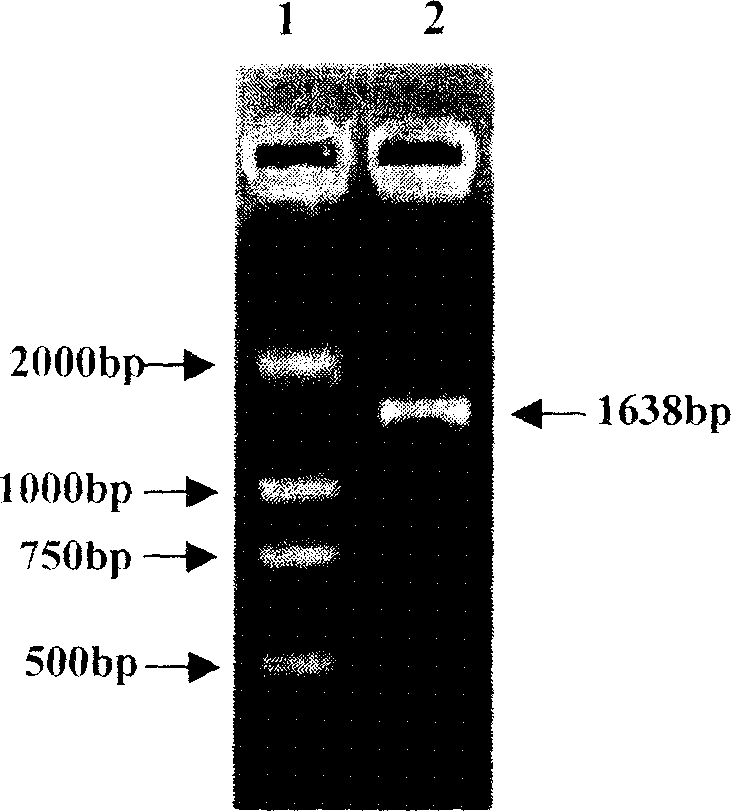
[0066] 3. Isolation of HSP65 structural gene: The HSP65 structural gene was isolated from BCG by PCR method. The 5' end primer and the 3' end primer used are respectively shown in SEQ ID NO: 6 and SEQ ID NO: 7.
[0067] PCR operating procedure
[0068] Add the following reagents to a 500 μl microcentrifuge tube:
[0069] Template cDNA (mmol / L) 5μl
[0070] 10× PCR buffer (containing m...
Embodiment 2
[0079] Example 2 Obtaining of multi-epitope hepatitis B virus core antigen gene
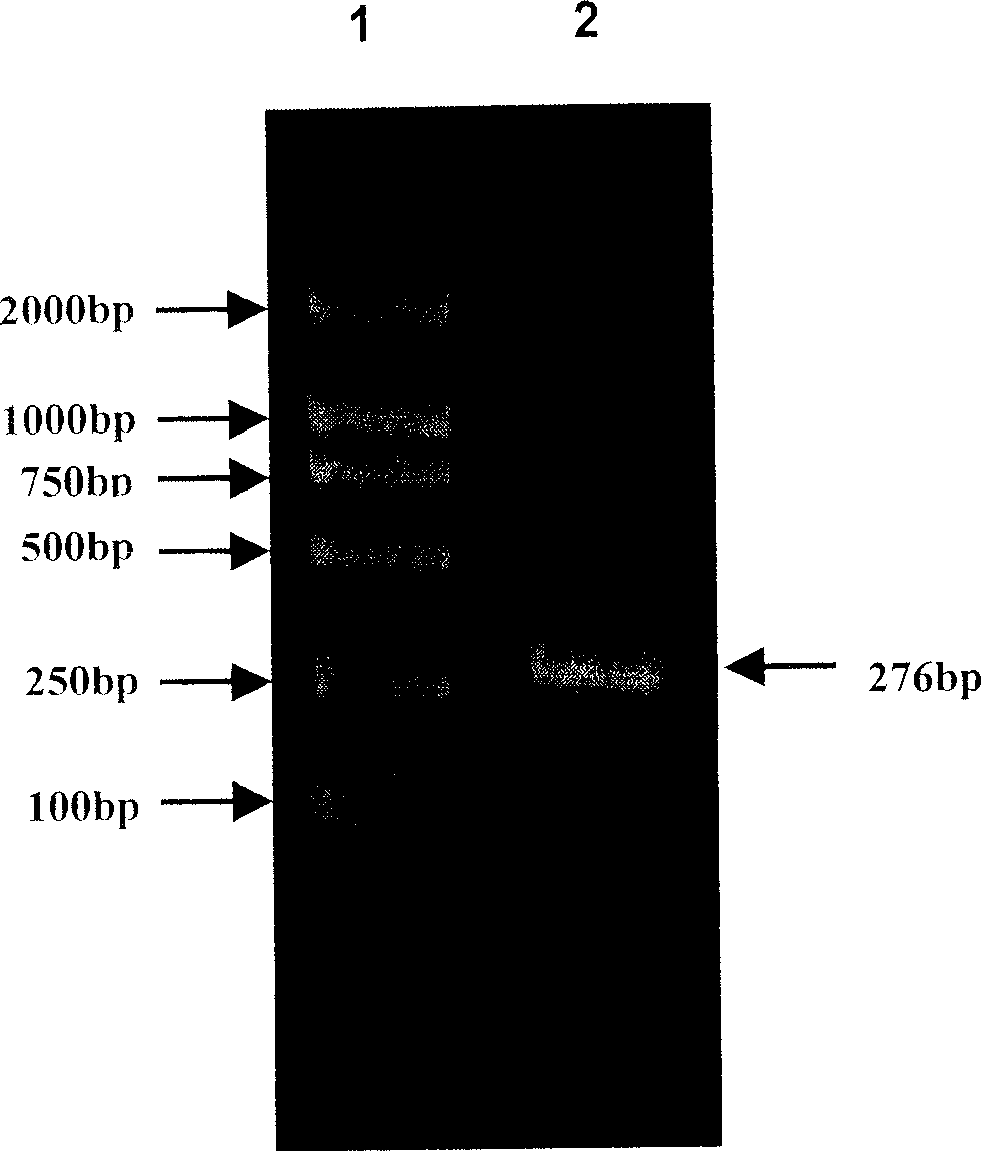
[0080] The multi-epitope HBV core antigen gene was synthesized by two rounds of PCR.
[0081] 1. The first round of PCR: the used primer 1 and primer 1' are shown in SEQ ID NO: 9 and SEQ ID NO: 10, respectively. The product obtained from the first round of PCR is shown in SEQ ID NO:11.
[0082] 2. The second round of PCR: the primers 2 and 2' used are shown in SEQ ID NO: 12 and SEQ ID NO: 13, respectively. The product obtained by the second round of PCR is shown as SEQ ID NO: 14, which represents the multi-epitope gene of the hepatitis B virus core antigen.
[0083] The PCR reaction conditions were: 94°C, 30"; 55°C, 1'; 72°C, 4', after 30 cycles, 72°C was extended for 10 minutes.
[0084] The PCR map is attached to the instructions figure 2 shown.
Embodiment 3
[0085] Example 3 Construction of HSP65-HBcAg fusion gene
[0086] 1. The heat shock protein 65 (HSP65) coding gene was digested with NcoI and EcoRI, and the multi-epitope hepatitis B virus core antigen gene was digested with EcoRI and HindIII at 37° C. for 2 hours. The digested products were separated by agarose gel electrophoresis. Electrophoresis conditions are: 1% agarose gel, 1×TAE buffer, 150-200mA, electrophoresis for 0.5-1 hour.
[0087] 20×TAE buffer: 0.8mol / L Tris base
[0088] 0.4mol / L NaOAc
[0089] 0.04mol / L Na 2 EDTA
[0090] Adjust the pH to 8.3 with glacial acetic acid.
[0091] 2. Observe under ultraviolet light and cut out the agarose gel containing the DNA band, freeze at -70°C for 15 minutes, and then melt the gel in a water bath at 65°C; add an equal volume of TE-saturated phenol, vigorously Shake for 30 seconds, then freeze at -70°C for 15 minutes; after thawing at room temperature, centrifuge at 12,000 rpm for 5 minute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com