Synthesis process of carbamido carboxymethyl chitosan
A technology of carboxymethyl chitosan and chitosan, which is applied in the field of synthesis of ureido carboxymethyl chitosan derivatives, can solve the problems of limited application range and poor water solubility of chitosan derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
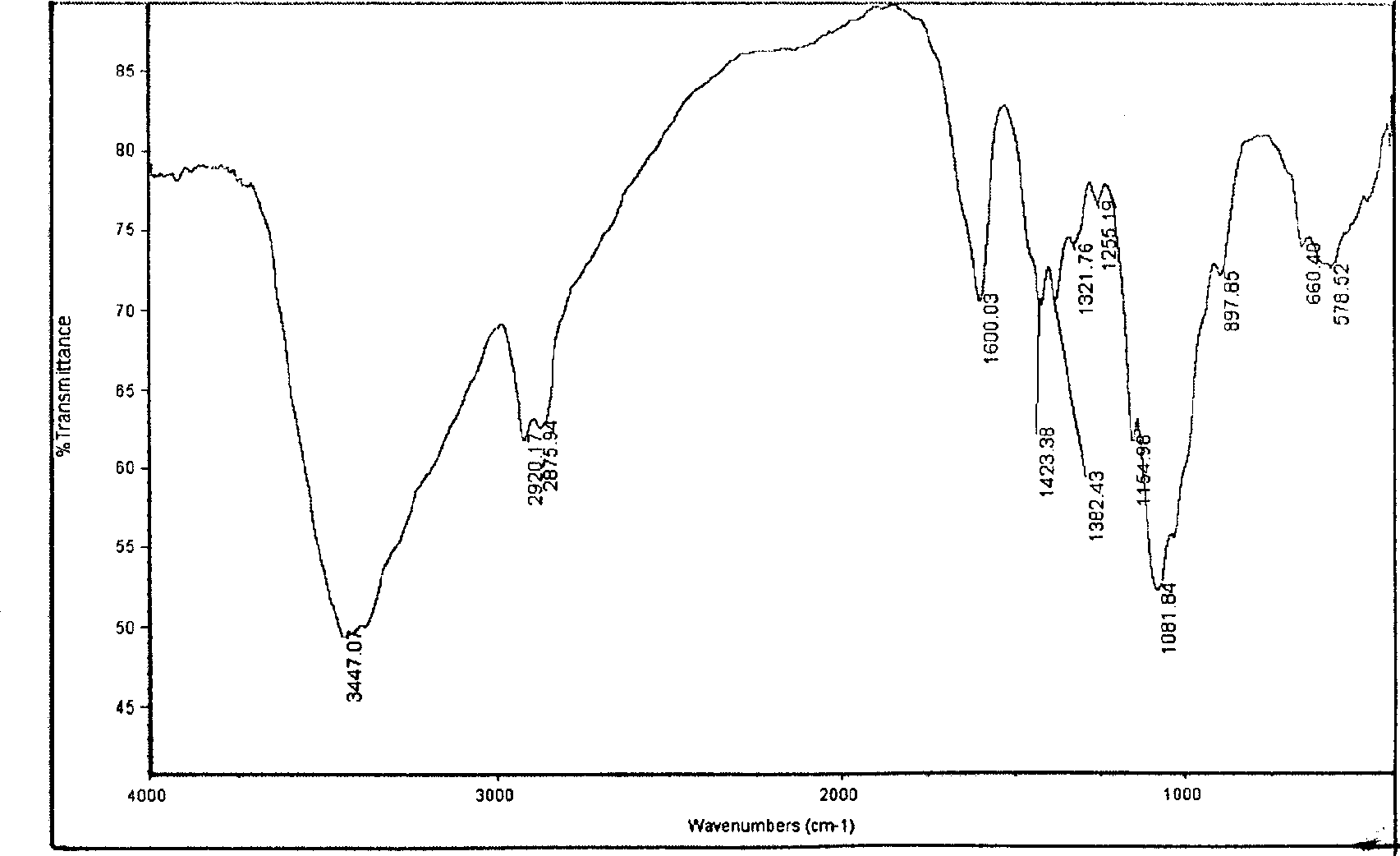
Image
Examples
Embodiment 1
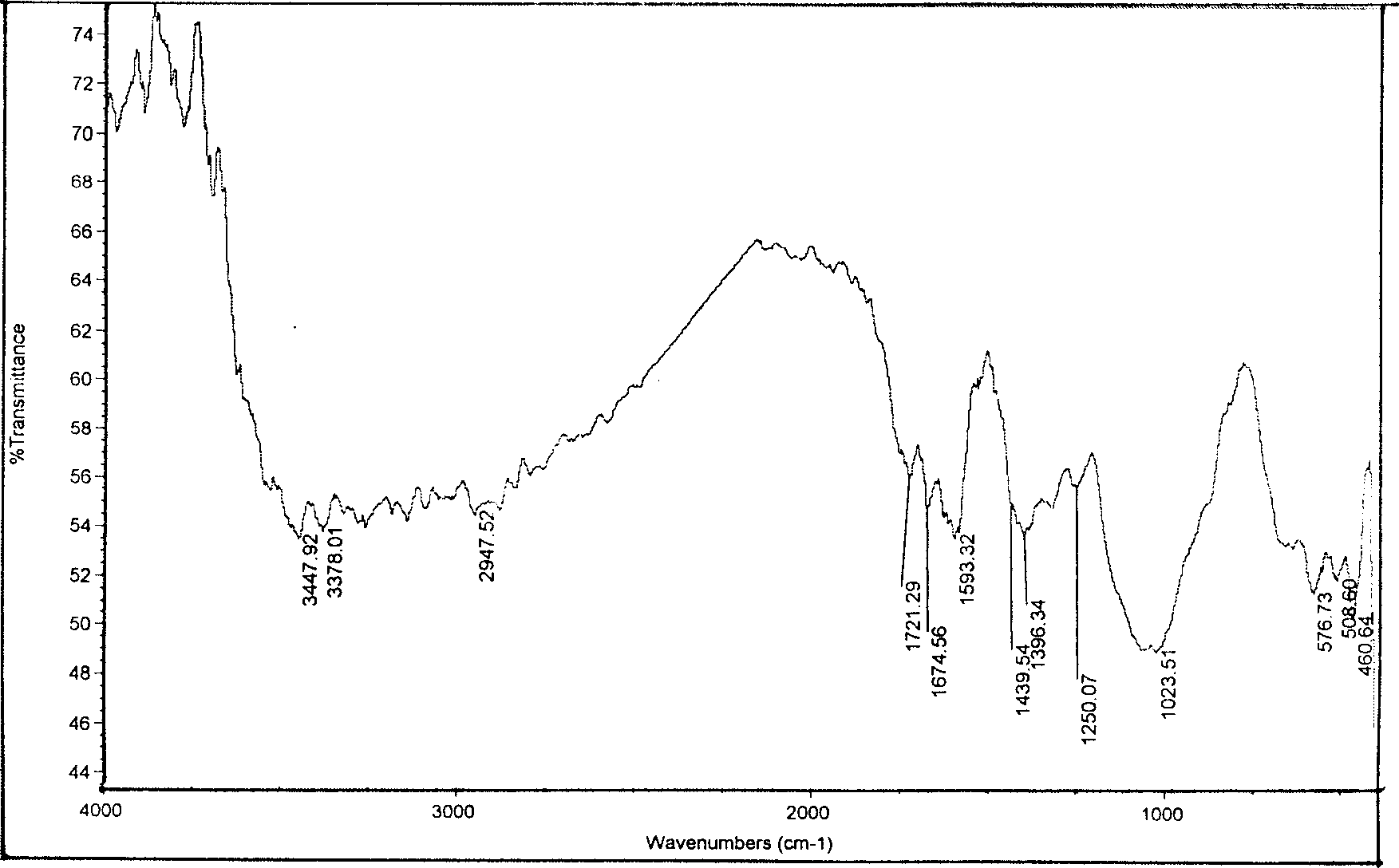
[0023] Example 1: under stirring, 1 gram of carboxymethyl chitosan was added into 10 ml of DMF and anhydrous acetic acid mixed solution (50:50) and placed overnight, and then reacted with 1 gram of phenylisocyanate at 100° C. for 3 hours, After the reaction is finished, it is filtered, washed with DMF, acetone and ethanol respectively, and freeze-dried under vacuum to obtain a phenylureido carboxymethyl chitosan derivative. The product was confirmed by infrared spectrum analysis, 1674.51cm -1 It is the absorption peak of urea carbonyl, 1439.54 and 1593.32cm -1 It is the characteristic absorption peak of benzene, proving the formation of the target compound.
Embodiment 2
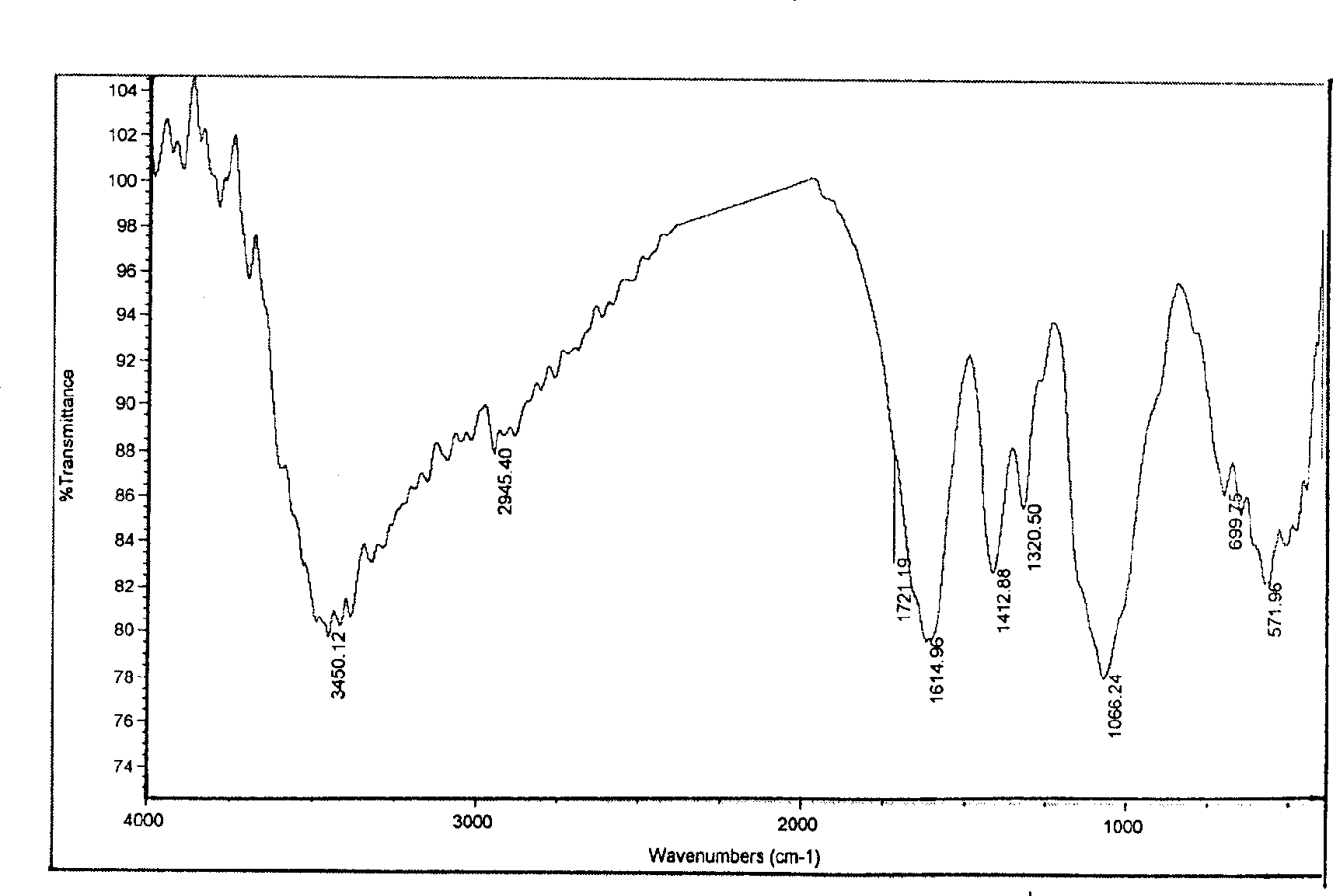
[0024] Example 2: under stirring, 1 gram of carboxymethyl chitosan was added to 20 ml of DMF and anhydrous acetic acid mixed solution (95: 5) and placed overnight, and then reacted with 3.16 gram of methylnaphthalene isocyanate at 110° C. 7 hours, after the reaction is finished, filter, then wash with DMF, acetone and ethanol respectively, and freeze-dry under vacuum to obtain menylureido carboxymethyl chitosan derivatives. The product was confirmed by infrared spectrum analysis, 1645.00cm -1 It is the absorption peak of urea carbonyl, 1395.68 and 1470.27cm -1 is the absorption peak of the naphthalene ring, proving the formation of the target compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com