Controlled release of highly soluble agents
A drug and sustained-release technology, applied in the field of delivering sustained-release preparations and treating glaucoma, can solve problems such as inappropriate preparations and administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
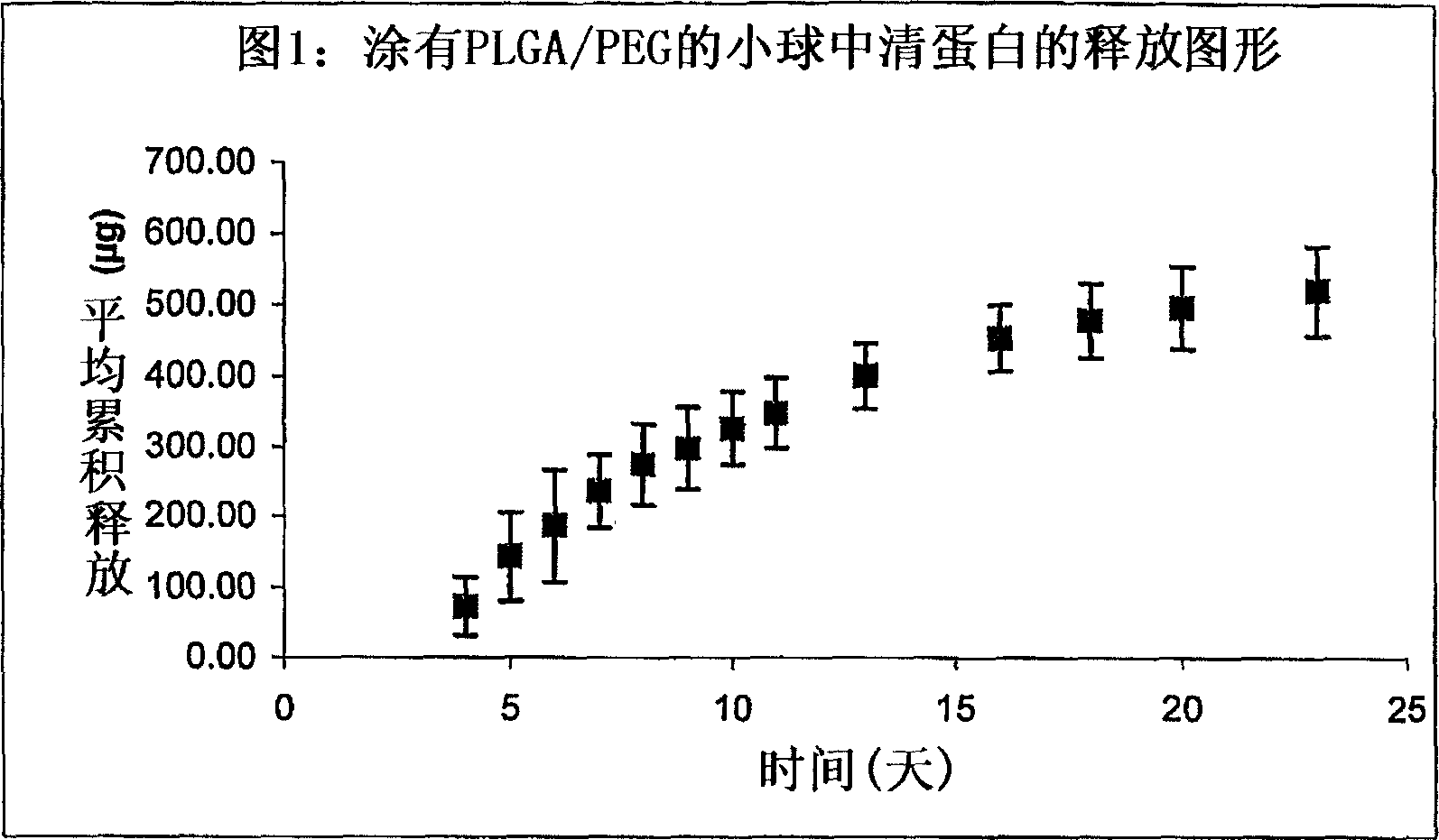
[0080] Particles (1.5 mm in diameter, 2.0 mg) comprising D,L-lactide-co-glycolide (PLGA) and bovine albumin (1:1, w:w) were manually compressed. The particles were then dip-coated in a solution of PLGA / PEG (polyethylene glycol) in acetone and air dried. The dried coated particles were then embedded in a silicone array and covered by silicone, leaving 0.59mm diameter pores uncovered by silicone to allow albumin to diffuse.
[0081] The particles were placed in 1.0 ml of phosphate buffer (pH 7.4) and tested for release at 37°C. Samples were taken periodically and analyzed for albumin by HPLC.
[0082] figure 1 Cumulative release profile of particles coated with PLGA / PEG(8 / 2) is shown.
Embodiment 2
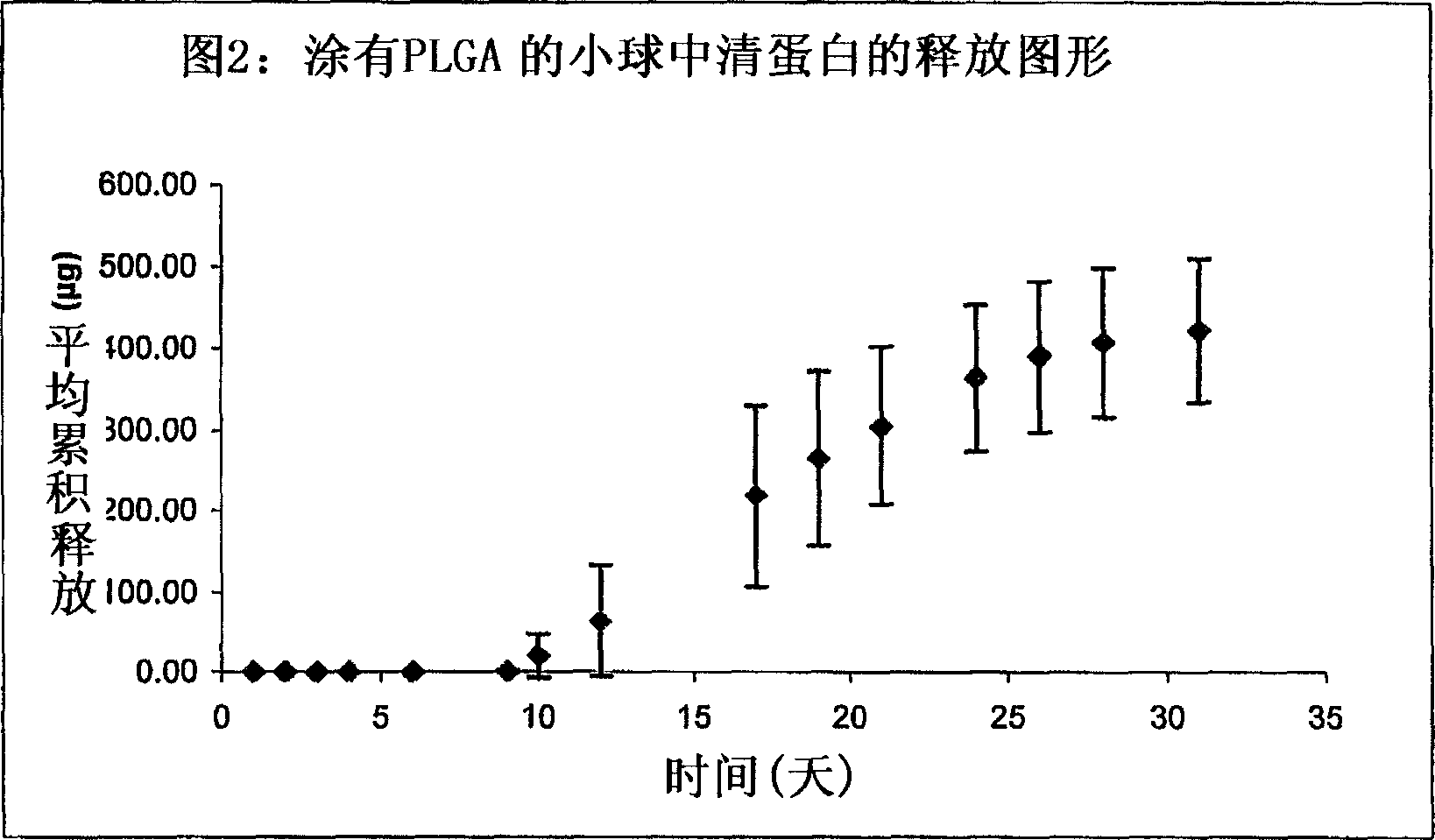
[0084]Particles (1.5 mm in diameter, 2.0 mg) comprising D,L-lactide-co-glycolide (PLGA) and bovine albumin (1:1, w:w) were manually compressed. The particles were then dip-coated in a solution of PLGA in acetone and air-dried. The dried coated particles were then embedded in a silicone array and covered by silicone, leaving 0.59mm diameter pores uncovered by silicone to allow albumin to diffuse.
[0085] The particles were placed in 1.0 ml of phosphate buffer (pH 7.4) and tested for release at 37°C. Samples were taken periodically and analyzed for albumin by HPLC.
[0086] figure 2 Cumulative release graph representing PLGA-coated particles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com