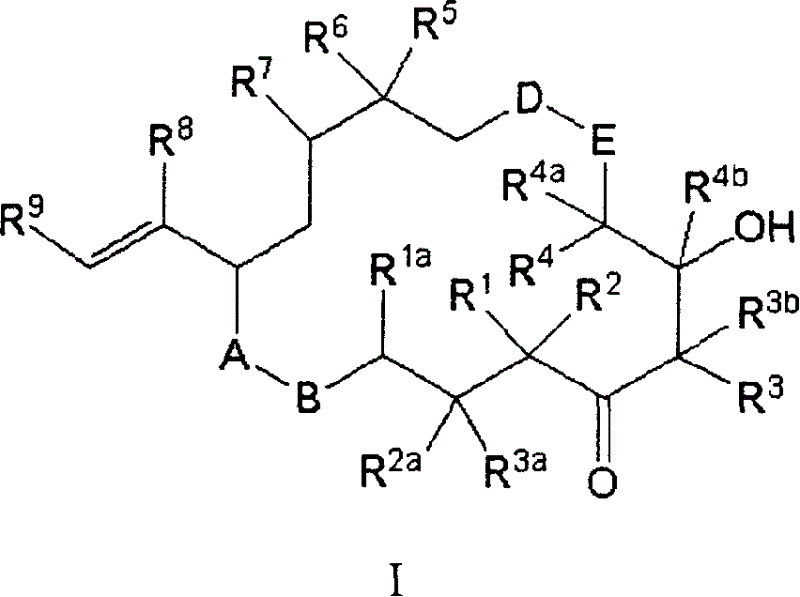
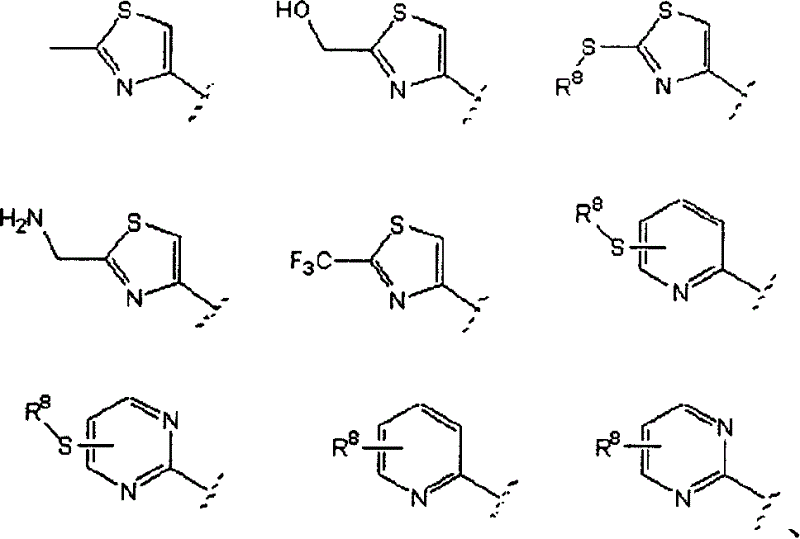
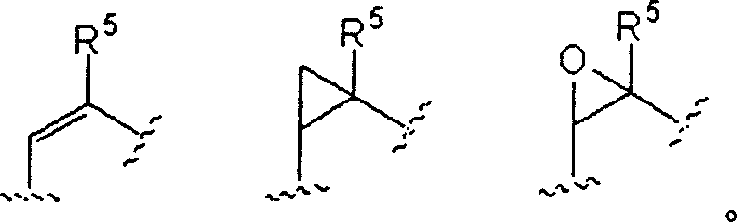
Aibomycin analogue, its preparation method, medicinal composition and use
A composition and drug technology, applied in the field of epothilone analogues, can solve problems such as weakening natural products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] 4-Oxo-pentanoyl chloride
[0180]
[0181] Dissolving 4-oxo-pentanoic acid in CH 2 Cl 2 , add 0.01 equivalent of DMF, and then add 1.1 equivalent of oxalyl chloride. The mixture was stirred at room temperature for 6 hours and concentrated to give the crude title compound which was used without further purification.
Embodiment 2
[0183] 1-(4-Benzyl-2-oxo-oxazolidin-3-yl)-pentan-1,4-dione
[0184]
[0185] 4-Benzyl-oxazolidin-2-one was dissolved in THF (tetrahydrofuran) at -78°C, and 1.1 equivalents of n-butyllithium dissolved in hexane was added. The mixture was stirred at -78°C for 10 minutes, followed by the addition of 1 equivalent of the product of Example 1 in THF. The resulting mixture was stirred for another 30 min and washed with NH 4 The reaction was quenched with Cl solution and extracted with EtOAc. use Na 2 SO 4 The EtOAc extracts were dried and concentrated. The residue was purified by silica gel chromatography to give the title compound.
Embodiment 3
[0187] 4-Benzyl-3-[3-(2-methyl[1,3]-dioxol-2-yl)-propionyl]-oxazolidin-2-one
[0188]
[0189] The product of Example 2 was dissolved in -40°C CH 2 Cl 2 1.2 equivalents of 1,2-bis-trimethylsiloxyethane and 0.05 equivalents of TMSOTf (trimethylsilyltrifluoromethanesulfonic acid) were added. Stir the mixture at -40°C for 6 hours, add 0.2 equivalent of Et 3 N(triethylammonium). The solvent was removed under reduced pressure to give the crude title compound which was used without further purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com