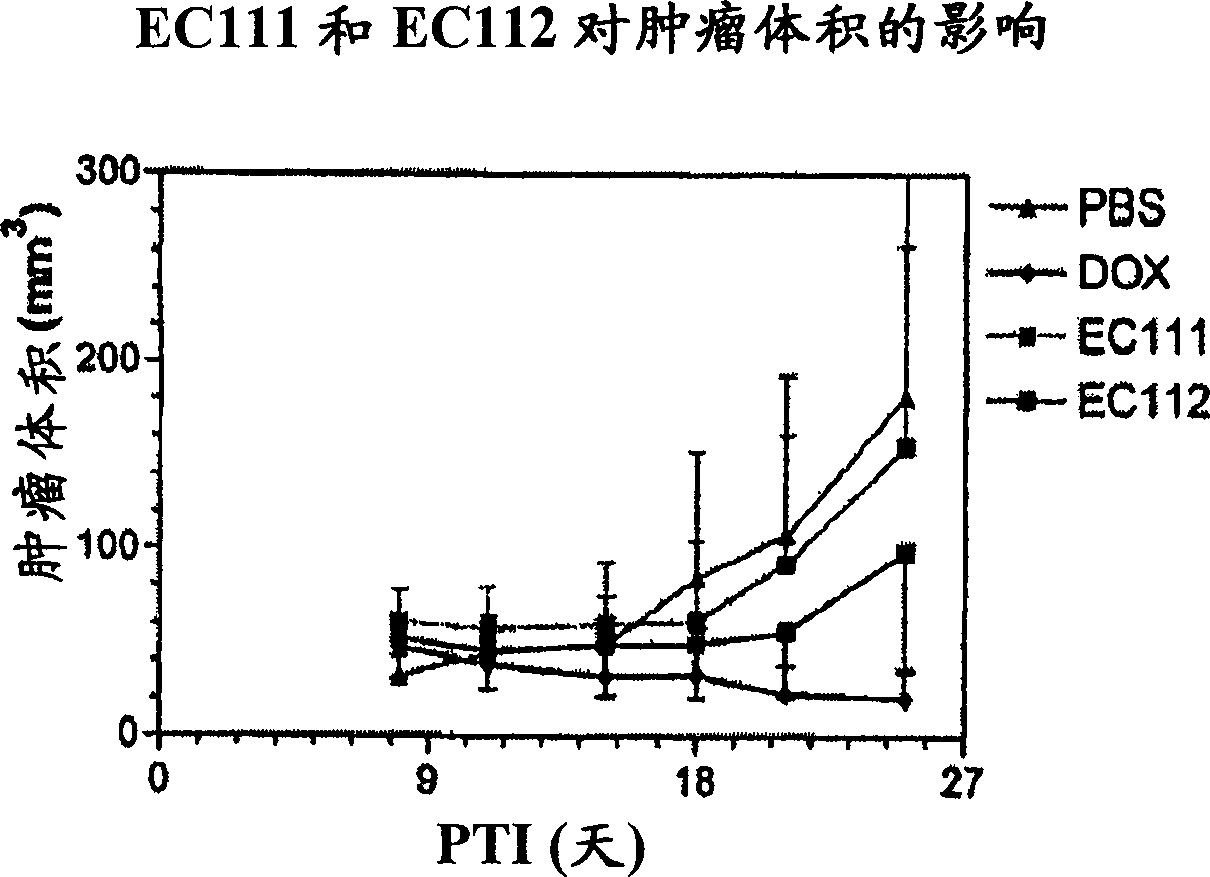
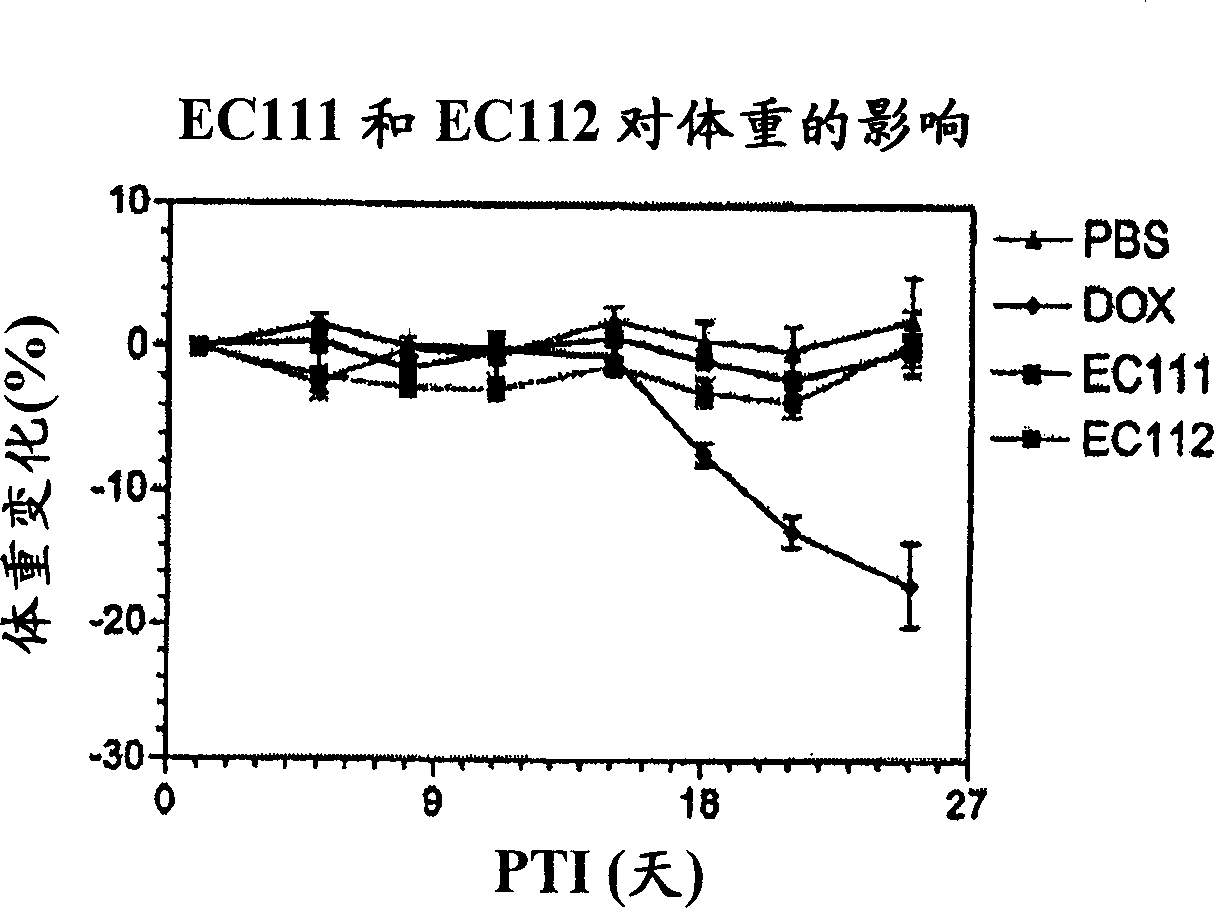
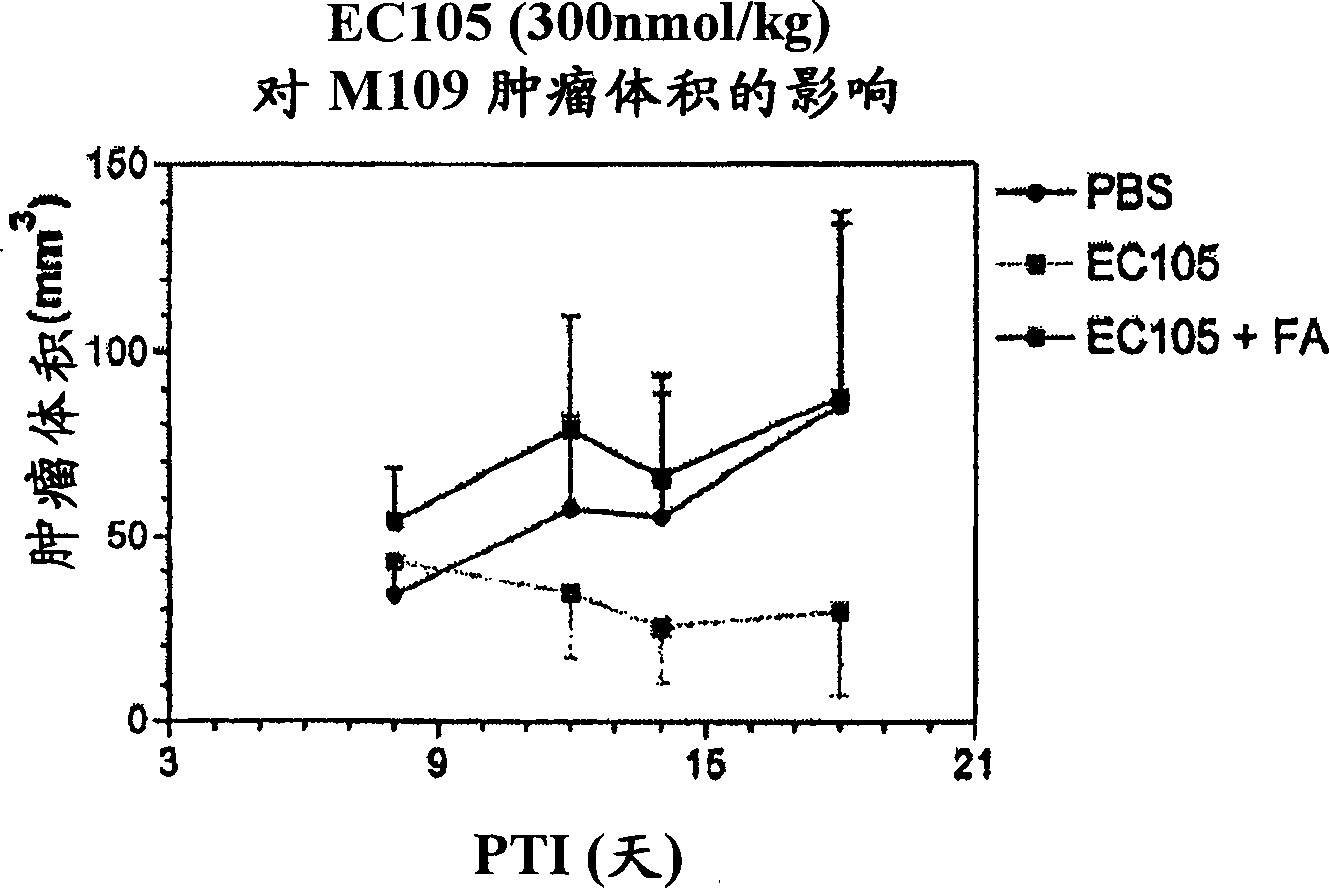
Vitamin receptor binding drug delivery conjugates
A technology of vitamin receptors and conjugates, applied in the field of targeted drug delivery compositions, which can solve the problems of tumor-specific antigen difficulties and complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0331]
[0332] According to the method described in P.Fuchs et al., J.Am.Chem.Soc., 1997, 119, 10004 diethylenetriamine folic acid, gamma-amide (DETA-folate), the disclosure of which is incorporated by reference In this article. This compound (100 mg) was dissolved in 2 ml of 0.1N HCl. The resulting solution was added to K 2 PtCl 4 (158 mg) in 1 ml of 0.1N HCl was stirred. 3ml of DMSO was added and stirred continuously for 3 days, the solution was filtered, and the filtrate was precipitated in acetonitrile to obtain 170mg of yellow powder;
[0333] MS (MALDI) 1249.92, 1286.27; 1 H NMR (D 2 O) δ1.05(t, 1H), 2.3(t, 2H), 3.1(t, 2H), 4.45(m, 1H), 6.65(d, 2H), 7.5(d, 2H), 8.65(s, 1H).
Embodiment 2a
[0335]
[0336] N 10 -Trifluoroacetyl-protected folate-containing peptidyl fragment N 10-TFA-Pte-Glu-Glu-Lys-OH was prepared by a polymer-supported continuous method with the Fmoc-strategy as outlined in Scheme 12. Synthesis was performed on acid-sensitive Fmoc-Lys(Boc)-Wang resin. PyBop was used as an activator to ensure efficient coupling with low equivalent weight amino acids. The Fmoc protecting group was removed after each coupling step under standard conditions (20% piperidine in DMF). Fmoc-Glu-OtBu and N 10 -TFA-Pte-OH was used as the protected amino acid building block. After the final assembly step, the peptide is cleaved from the polymer support by treatment with trifluoroacetic acid, ethanedithiol and triisopropylsilane. This reaction also results in simultaneous removal of the t-Bu and t-Boc protecting groups. The crude peptide was purified by preparative HPLC to give N 10 - TFA salt of TFA-Pte-γGlu-γGlu-Lys-OH. A solution of 81 mg (0.1 mmol) of the resu...
Embodiment 2b
[0339]
[0340] N 10 -Trifluoroacetyl-protected folate-containing peptidyl fragment N 10 - TFA-Pte-Glu-Cys-OH was prepared by a polymer-supported sequential method with the Fmoc-strategy, as outlined in Scheme 12 and described in Example 2a. Reaction of cystamine with mitomycin A (see Matsui et al., J.Antibiot.21, 189-198 (1968); Vias et al., J.Org.Chem.51, 4307-4309 (1986)) gives Disulfides of mitomycin C derivatives with free amino groups, which are coupled to levulinic acid followed by subsequent carbonyl reaction with maleimide-derived acylhydrazides. The resulting Michael acceptor with N 10 - TFA-Pte-Glu-Cys-OH reaction, after removal of the trifluoroacetyl protecting group with aqueous ammonium hydroxide solution (pH=10.0), precipitation from acetonitrile to give the final conjugate;
[0341] MS (MALDI) 1059.04, 1148.44, 1225.32, 1300.8; 1 H NMR (D 2 O) δ1.8(d, 2H), 1.9(s, 1H), 2.3(q, 1h), 2.45(q, 1H), 2.9(t, 1H), 3.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com