Oral medicinal formulation of moxifloxacin and its preparation method
A pharmaceutical preparation, the technology of moxifloxacin hydrochloride, which is applied in the field of pharmaceutical preparations and its preparation, can solve the problems of red impurities and achieve the effect of maintaining yellow color, good hardness and ensuring quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
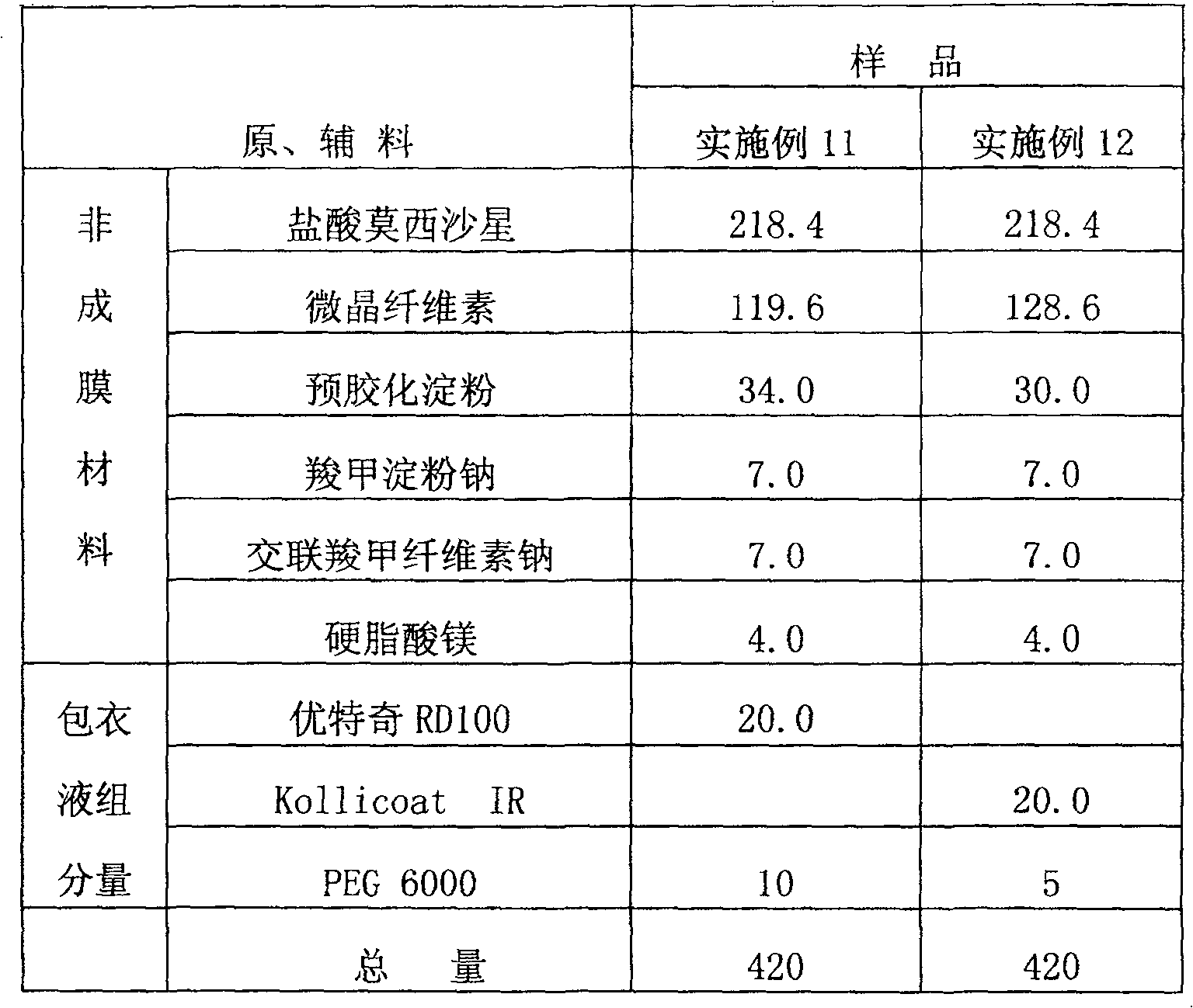
[0065] Components: Moxifloxacin Hydrochloride 218.4mg
[0066] Microcrystalline cellulose 124.6mg
[0067] Pregelatinized starch 37.0mg
[0068] Sodium starch glycolate 7.0mg
[0069] Croscarmellose Sodium 7.0mg
[0070] Magnesium stearate 4.7mg
[0071] HPMC(90SH 100) 1.3mg
[0072] Total weight 400.0mg
[0073] Take the above-mentioned ingredients and the auxiliary materials (except magnesium stearate and HPMC) and mix them thoroughly, use 1.0% HPMC aqueous solution to make granules, add magnesium stearate and mix evenly after drying, and press tablets to obtain. The punch and die used in tablet pressing are made of stainless steel.
[0074] The prepared tablet cores can be conventionally coated, or they can be directly sealed and packaged without coating.
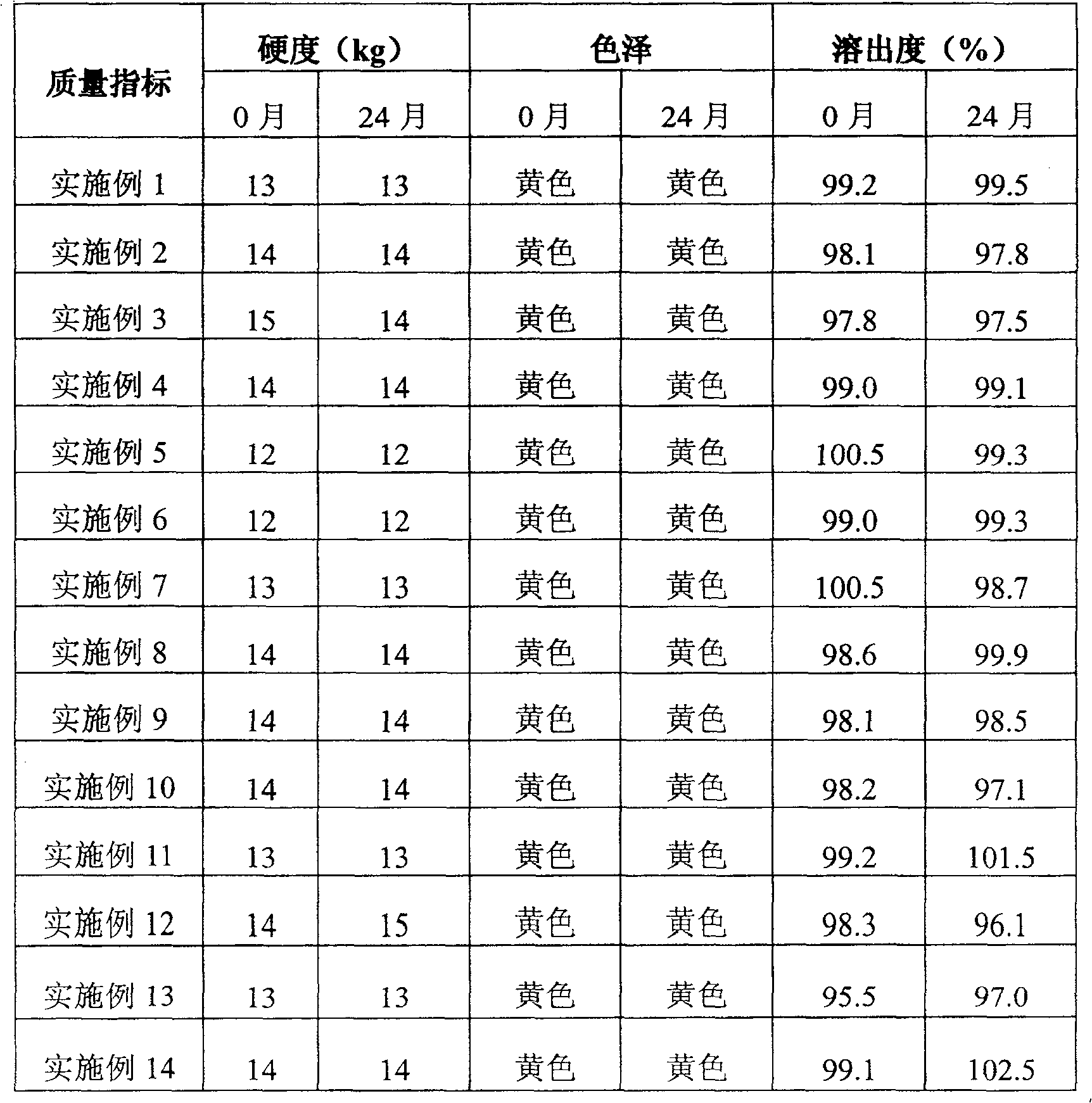
[0075] The quality of the tablets was examined immediately after the preparation was completed and two years later. The results are shown in Table 6.
Embodiment 2
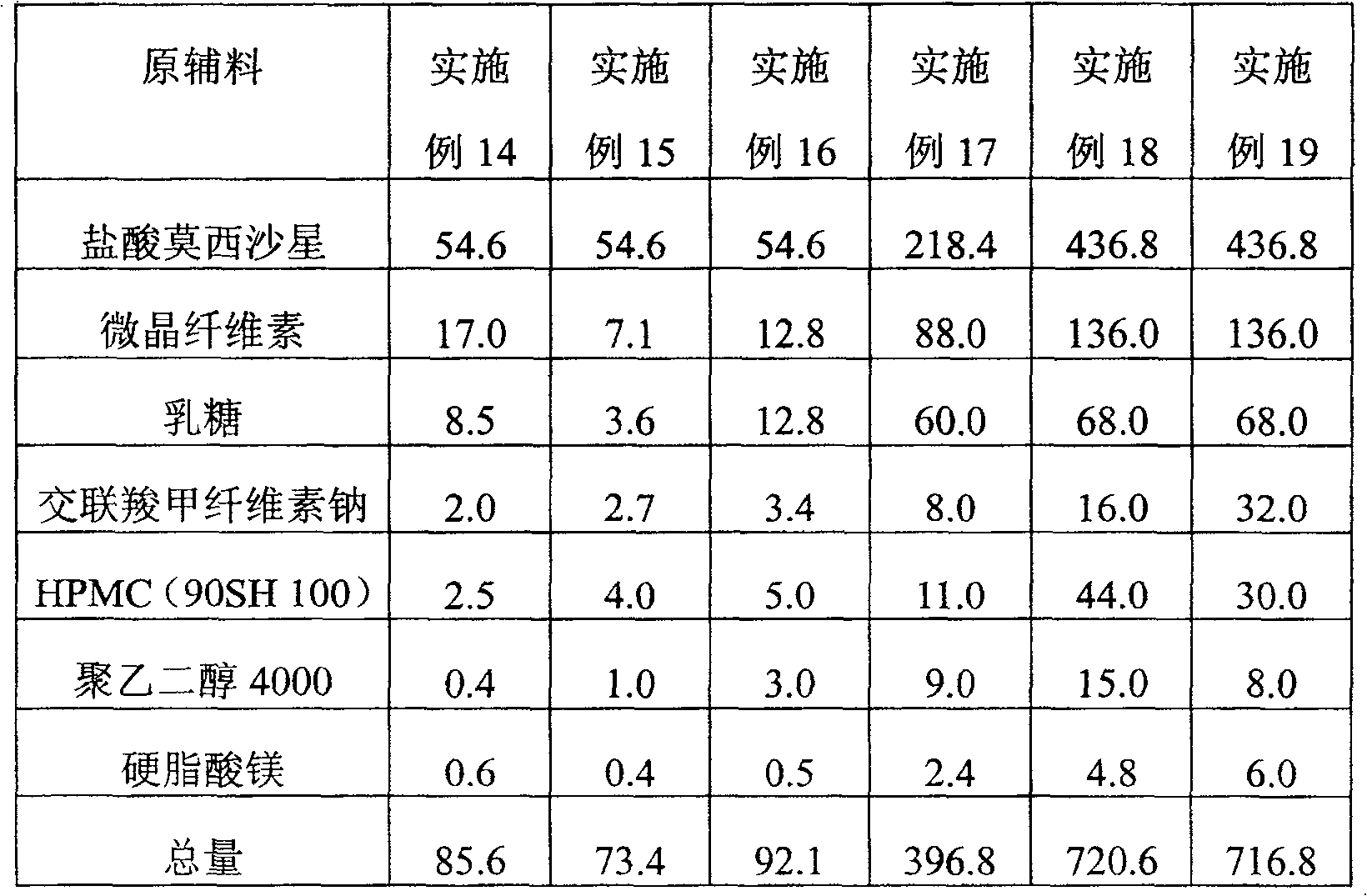
[0077] Components: Moxifloxacin Hydrochloride 218.4mg
[0078] Microcrystalline cellulose 124.6mg
[0079] Pregelatinized starch 34.3mg
[0080] Sodium starch glycolate 7.0mg
[0081] Croscarmellose Sodium 7.0mg
[0082] Magnesium stearate 4.7mg
[0083] HPMC(90SH 100) 4.0mg
[0084] Total weight 400.0mg
[0085] Take the above-mentioned ingredients and auxiliary materials (except magnesium stearate and HPMC) and mix them thoroughly, use 3.0% HPMC dilute ethanol solution (dilute ethanol is 50% ethanol) to make granules, and add magnesium stearate to mix after drying. Press the tablet and get it. The punch and die used in tablet pressing are made of stainless steel.
[0086] The prepared tablet core can be coated by a conventional method, or it can be directly sealed and packaged without coating.
[0087] The quality of the tablets was examined immediately after the preparation was completed and two years later, and the results are shown in Table 6.
Embodiment 3
[0089] Components: Moxifloxacin Hydrochloride 218.4mg
[0090] Microcrystalline cellulose 124.6mg
[0091] Pregelatinized starch 31.5mg
[0092] Sodium starch glycolate 7.0mg
[0093] Croscarmellose Sodium 7.0mg
[0094] Magnesium stearate 4.3mg
[0095] HPMC(90SH 100) 7.2mg
[0096] Total weight 400.0mg
[0097] Take the above-mentioned ingredients and the auxiliary materials (except magnesium stearate and HPMC) and mix them thoroughly, prepare granules with 4.5% HPMC aqueous solution, add magnesium stearate and mix evenly after drying, and then press tablets to obtain. The punch and die used for tableting are made of stainless steel.
[0098] The prepared tablet core can be coated by a conventional method, or it can be directly sealed and packaged without coating.
[0099] The quality of the tablets was examined immediately after the preparation was completed and two years later. The results are shown in Table 6.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com