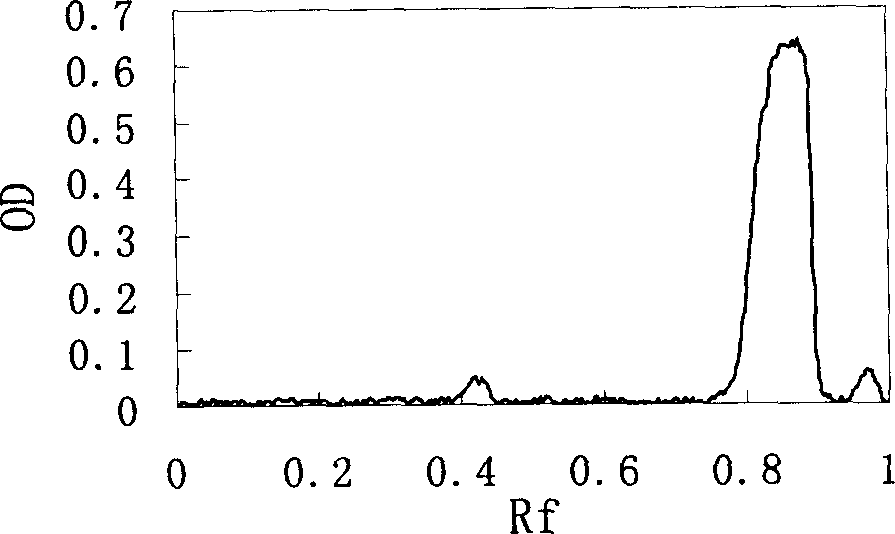
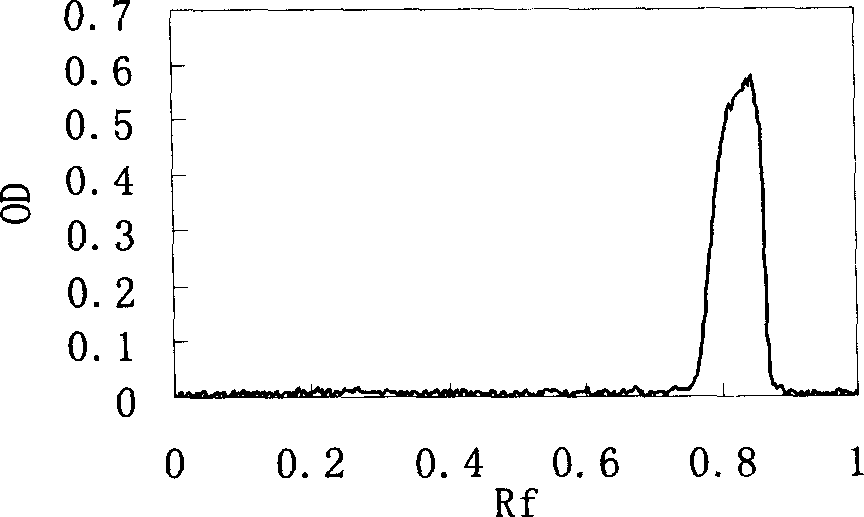
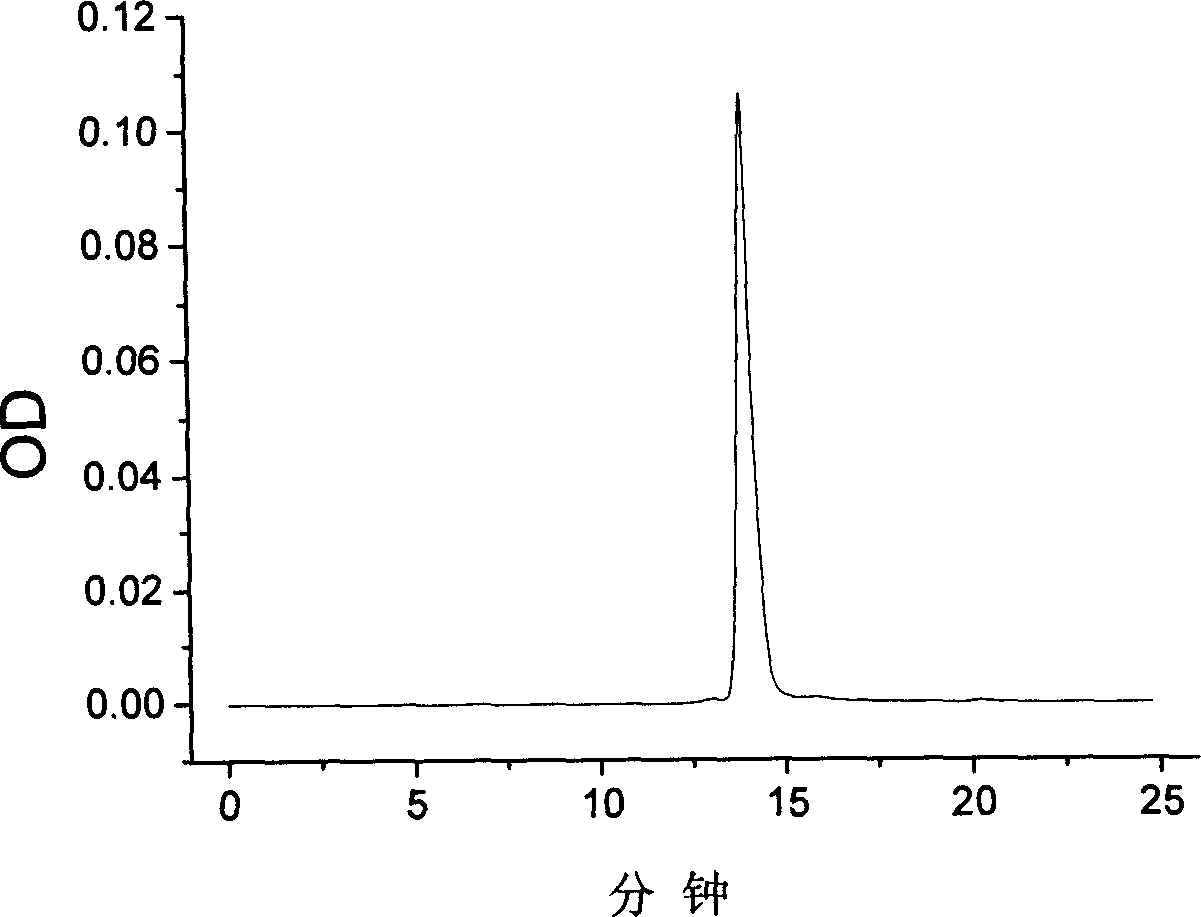
Hemoglobin conjugate and its preparing method and use
A technology for hemoglobin and conjugates, applied in the field of hemoglobin conjugates, can solve the problems of short retention time, large gap in blood function, limitations, etc., and achieve the effects of enhancing stability, prolonging circulatory half-life, and avoiding aggregation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Preparation of pure matrix-free bovine hemoglobin by electrophoresis
[0036] Take a certain volume of washed fresh bovine blood red blood cells, and use twice the volume of cold swelling solution (20mmol / L KH containing 0.6% NaCl 2 PO 4 / Na 2 HPO 4 (PBS) buffer, pH7.4) suspension, shake at 4°C for 1h; pump 20mmol / LPBS buffer (pH7.4) twice the volume of red blood cells at a rate of 10% of the total volume of the solution per minute, adjust NaCl after shaking for 1h The salt concentration is 0.9%, and the red blood cell swelling and rupture solution is obtained. The rupture fluid was pretreated by a Millipore Pellicon cross-flow membrane filtration system. First, use 0.22μm membrane microfiltration to remove cell debris and macromolecular impurities, and then use membrane ultrafiltration with a molecular weight cut-off of 10KD to remove small molecular impurities. The obtained hemoglobin solution was further purified by DEAE Sepharose Fast Flow anion e...
Embodiment 2
[0037] Embodiment 2: Preparation of chromatographically pure matrix-free porcine hemoglobin
[0038] Take a certain volume of washed fresh porcine red blood cells, and use twice the volume of cold swelling solution (20mmol / L KH containing 0.6% NaCl 2 PO 4 / Na 2 HPO 4 (PBS) buffer, pH7.4) suspension, shake at 4°C for 1h; pump 20mmol / LPBS buffer (pH7.4) twice the volume of red blood cells at a rate of 10% of the total volume of the solution per minute, adjust NaCl after shaking for 1h The salt concentration is 0.9%, and the red blood cell swelling and rupture solution is obtained. The rupture fluid was pretreated by a Millipore Pellicon cross-flow membrane filtration system. First, use 0.45μm membrane microfiltration to remove cell debris and macromolecular impurities, and then use membrane ultrafiltration with a molecular weight cutoff of 30KD to remove small molecule impurities. The obtained hemoglobin solution was further purified by Q Sepharose BigBeads anion exchange c...
Embodiment 3
[0039] Example 3: One-step cross-linking of human hemoglobin and human serum albumin by m-maleimide benzoic acid-N-hydroxysuccinimide ester (MBS)
[0040] Since the sulfhydryl group of the HSA molecule will react with MBS, thereby affecting the coupling reaction. Therefore, blocking of remaining sulfhydryl groups is required prior to activation of HSA with MBS. 0.2 ml of 30 mM iodoacetamide was slowly added to 10 ml of 5 mg / ml HSA solution (HEPES buffer at pH 7.8), and reacted at room temperature for 20 minutes. Subsequently, 0.5 ml of 30 mM MBS solution (dissolved in dimethylformamide) was added and reacted at room temperature for 30 minutes. The reaction mixture was passed through a Sephadex G-25 gel filtration column to remove excess MBS and iodoacetamide, and the protein fraction was collected. Because the hemoglobin molecule has a reactive thiol group (the sulfhydryl group of β-93 cysteine), the activated HSA can directly react with the hemoglobin molecule. The collect...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap