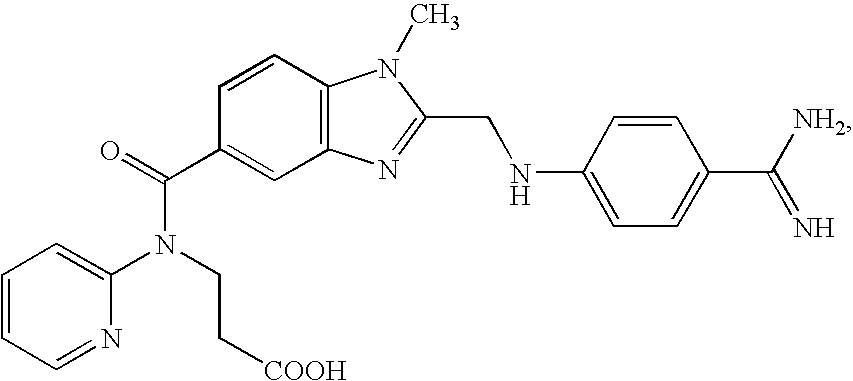
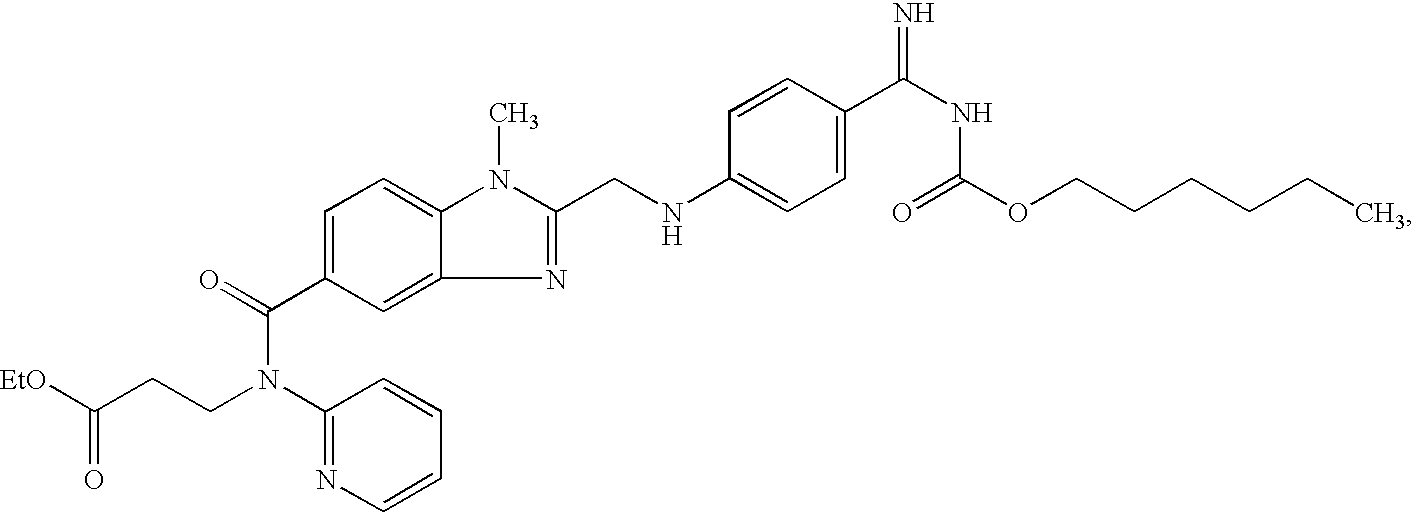
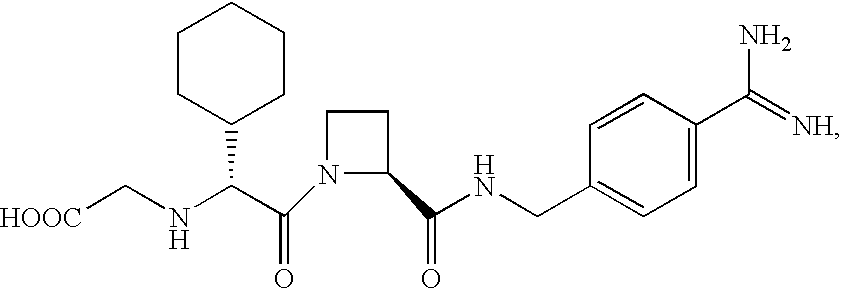
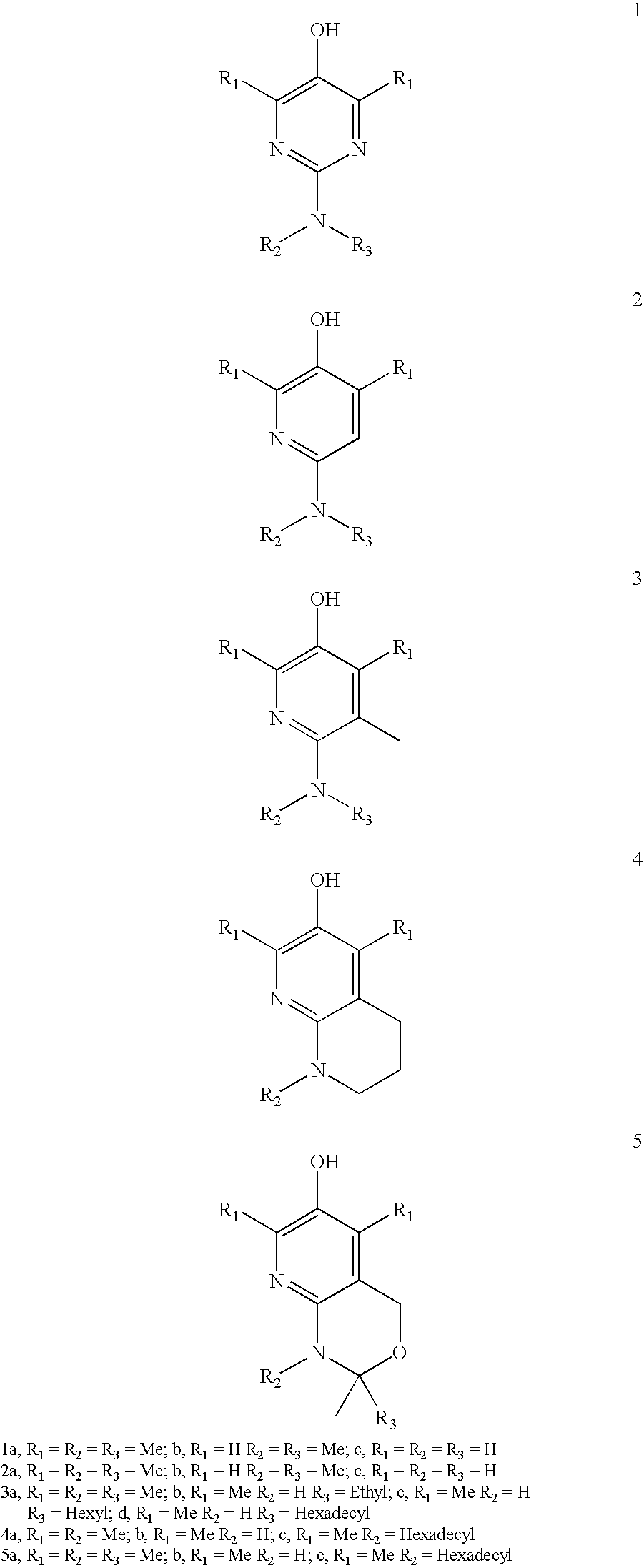
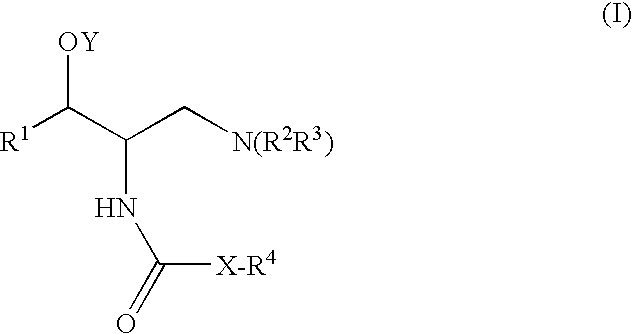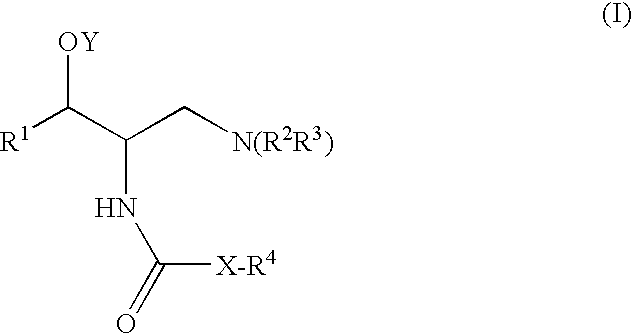Patents
Literature
125 results about "Hemeprotein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
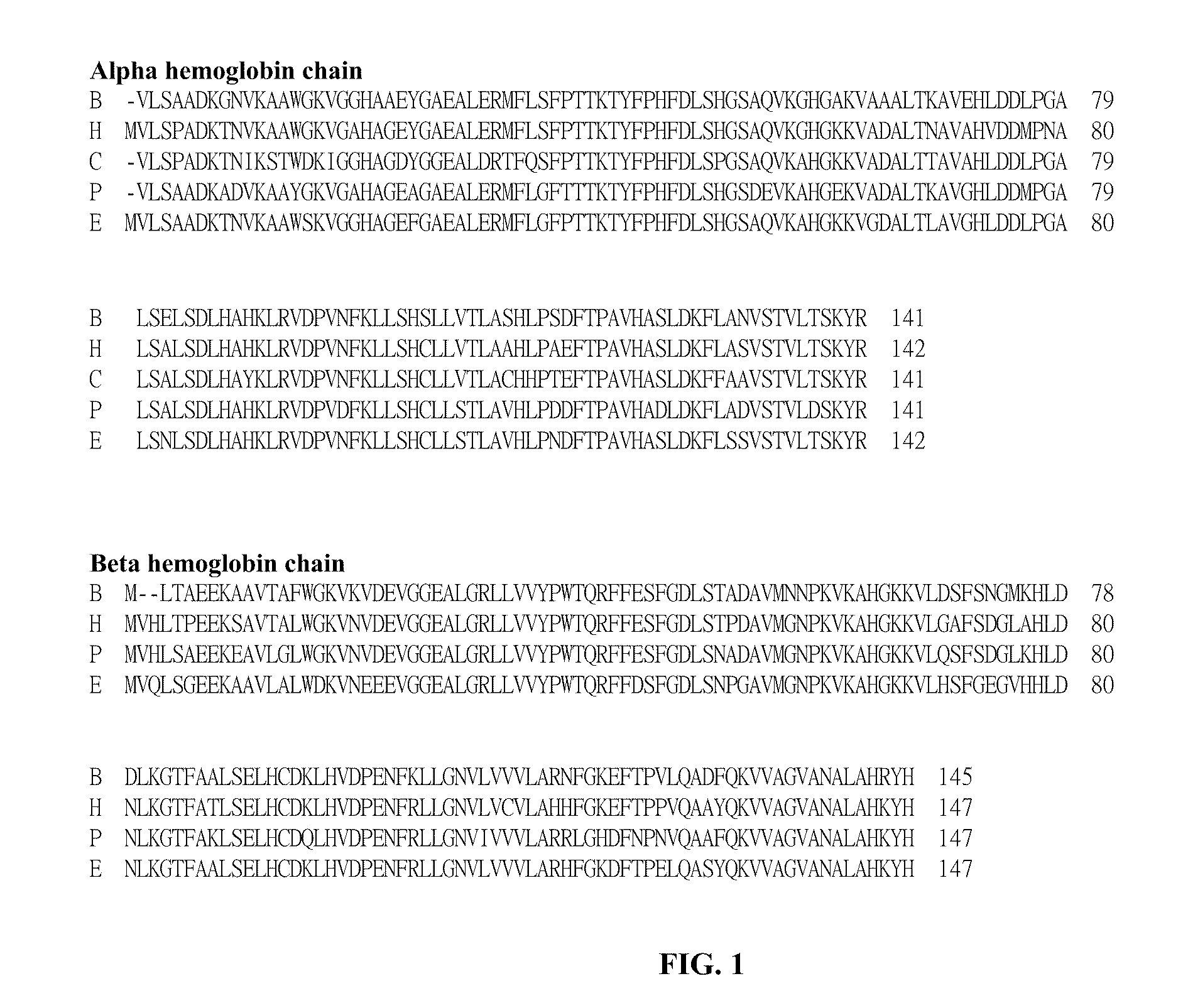
A hemeprotein (or haemprotein; also hemoprotein or haemoprotein), or heme protein, is a protein that contains a heme prosthetic group. They are a large class of metalloproteins. The heme group confers functionality, which can include oxygen carrying, oxygen reduction, electron transfer, and other processes. Heme is bound to the protein either covalently or noncovalently or both.
Method for the preparation of a heat stable oxygen carrier-containing pharmaceutical composition
ActiveUS7932356B1Increase oxygenationHigh sensitivityPeptide/protein ingredientsMammal material medical ingredientsArteriolar VasoconstrictionCross-link
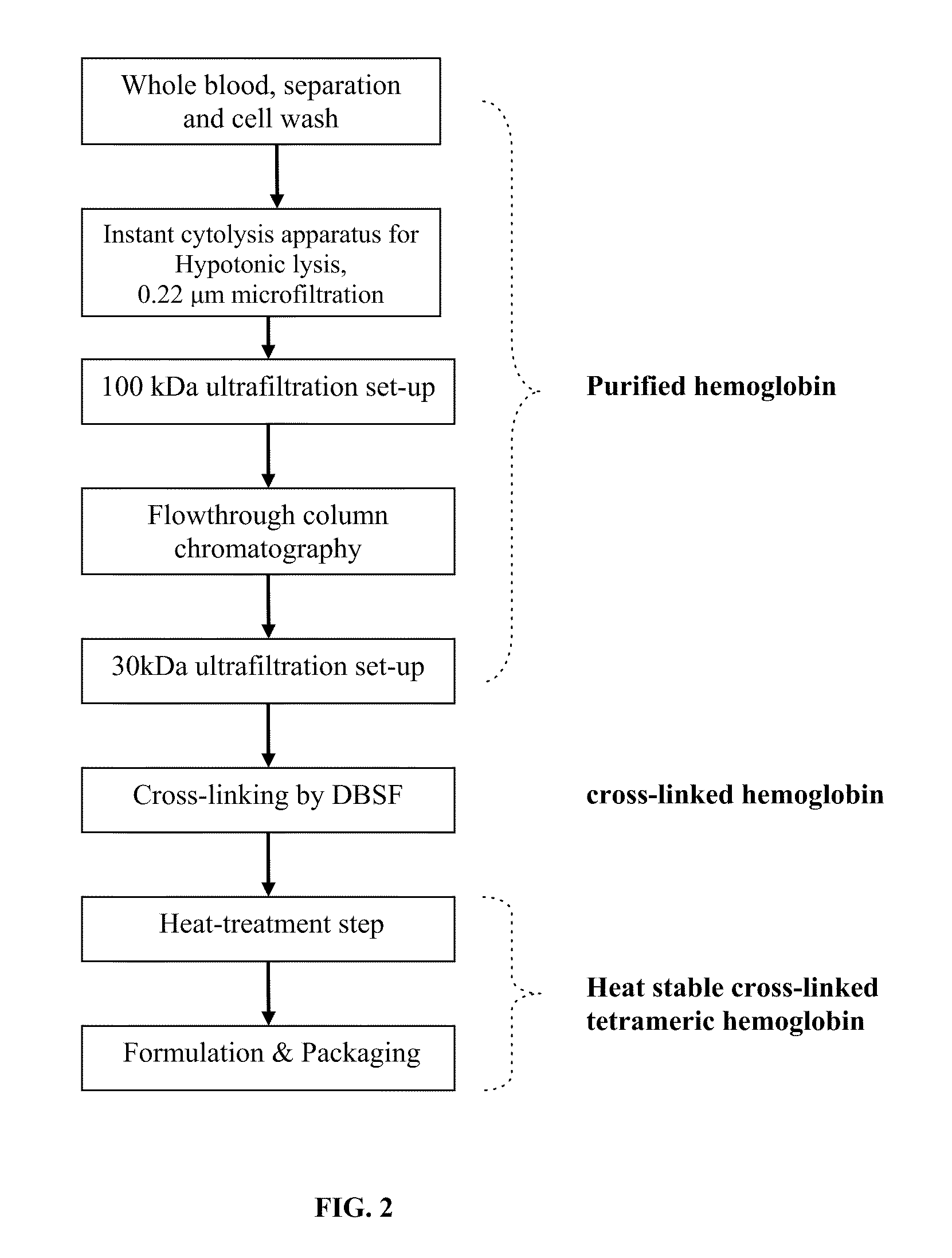
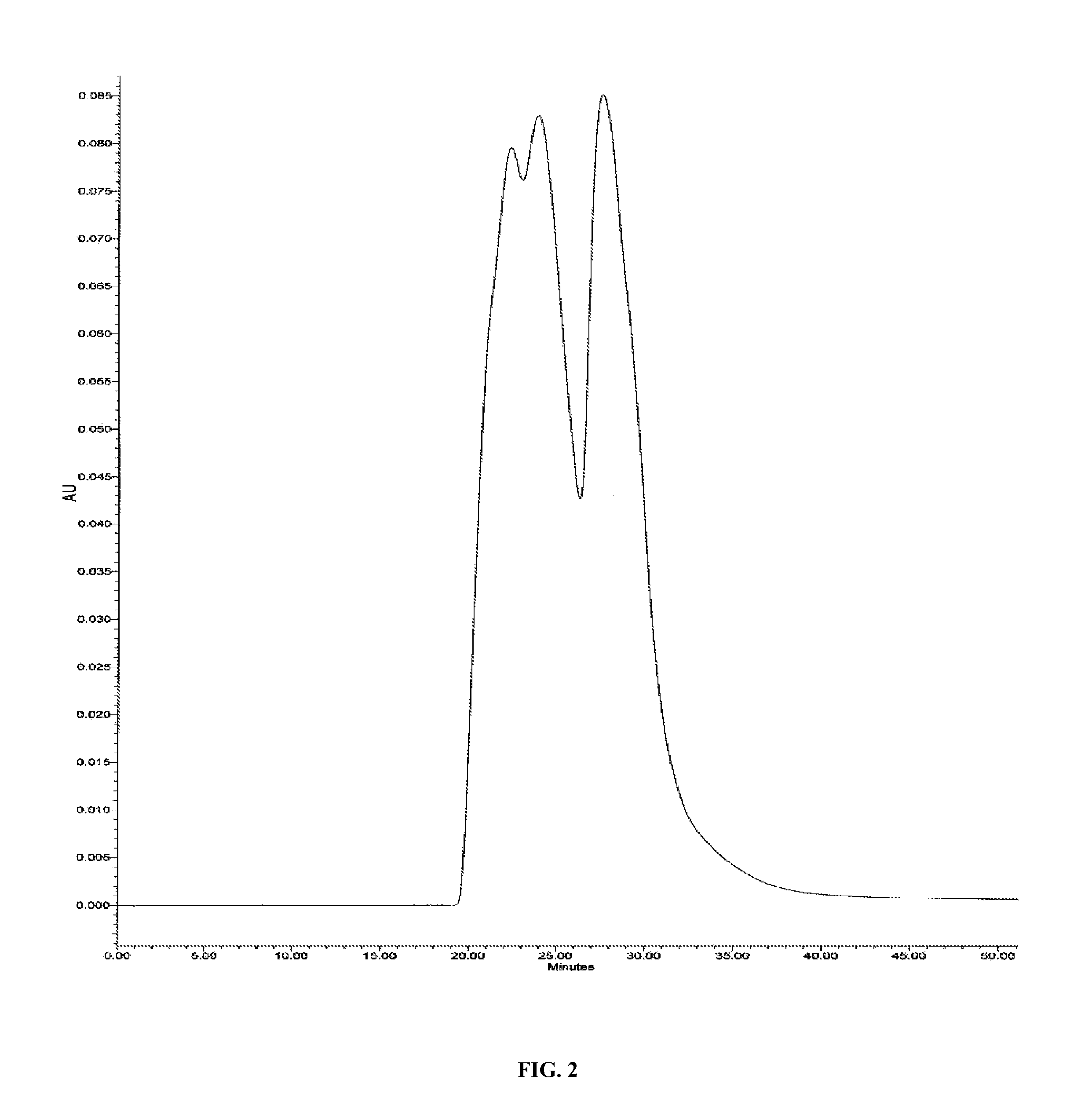
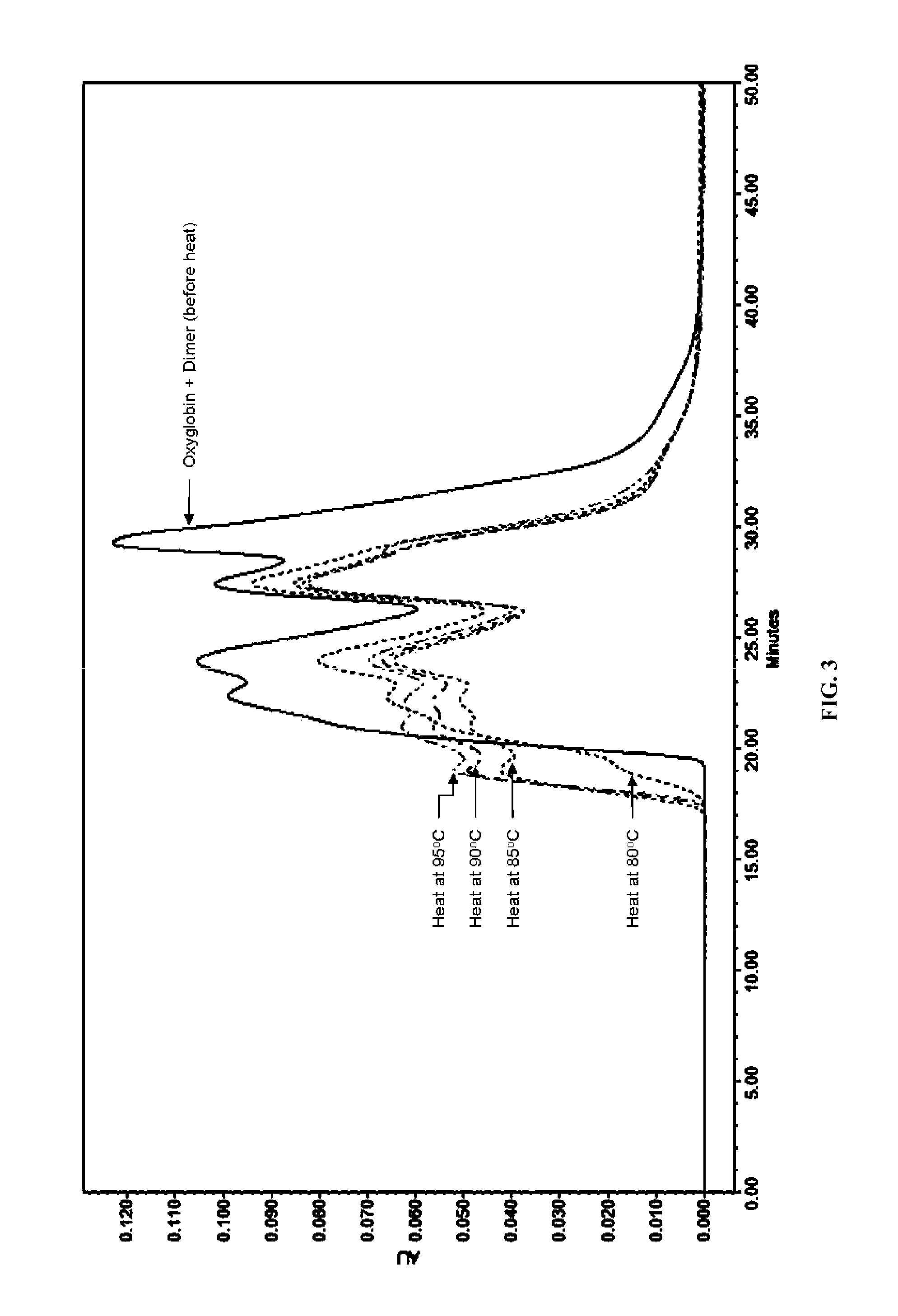
A highly purified and heat stable cross-linked nonpolymeric tetrameric hemoglobin suitable for use in mammals without causing renal injury and vasoconstriction is provided. A high temperature and short time (HTST) heat processing step is performed to remove undesired dimeric form of hemoglobin, uncross-linked tetrameric hemoglobin, and plasma protein impurities effectively. Addition of N-acetyl cysteine after heat treatment and optionally before heat treatment maintains a low level of met-hemoglobin. The heat stable cross-linked tetrameric hemoglobin can improve and prolong oxygenation in normal and hypoxic tissue. In another aspect, the product is used in the treatment of various types of cancer such as leukemia, colorectal cancer, lung cancer, breast cancer, liver cancer, nasopharyngeal carcinoma and esophageal cancer. Another application is heart preservation in situations where there is a lack of oxygen supply in vivo, such as in heart transplant or oxygen-deprived heart.
Owner:BILLION KING INT
Size-selective hemoperfusion polymeric adsorbents
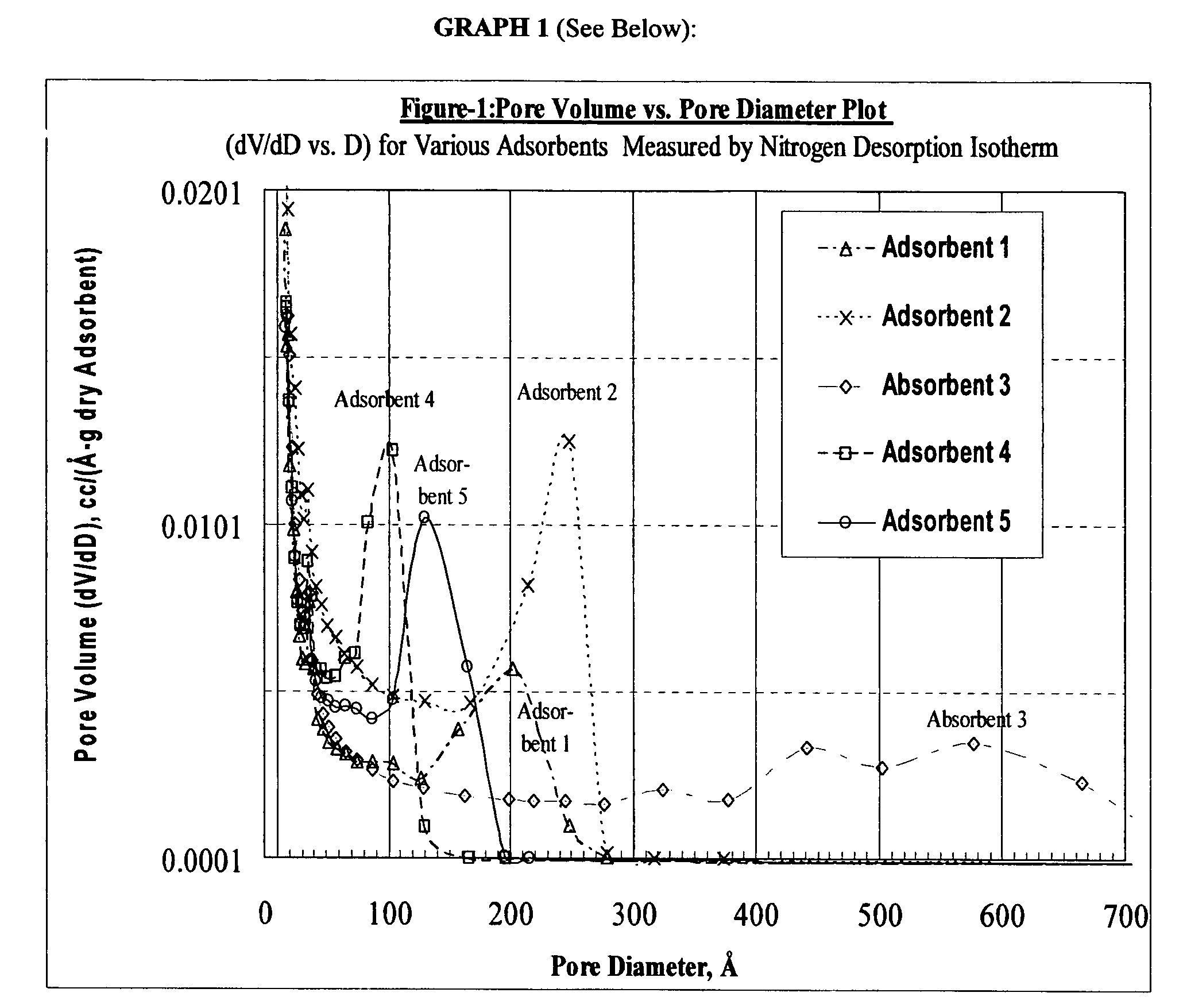
Size-selective hemocompatible porous polymeric adsorbents are provided with a pore structure capable of excluding molecules larger than 50,000 Daltons, but with a pore system that allows good ingress and egress of molecules smaller than 35,000 Daltons. The pore system in these porous polymeric adsorbents is controlled by the method of synthesis so that 98% of the total pore volume is located in pores smaller than 300 Angstroms (Å) in diameter with a working pore size range within 100 to 300 Å in diameter. The porous polymeric adsorbents of this invention are very selective for extracting midsize proteins, such as cytokines and β2-microglobulin, from blood and other physiologic fluids while keeping the components required for good health such as cells, platelets, albumin, hemoglobin, fibrinogen, and other serum proteins intact.
Owner:CYTOSORBENTS CORP
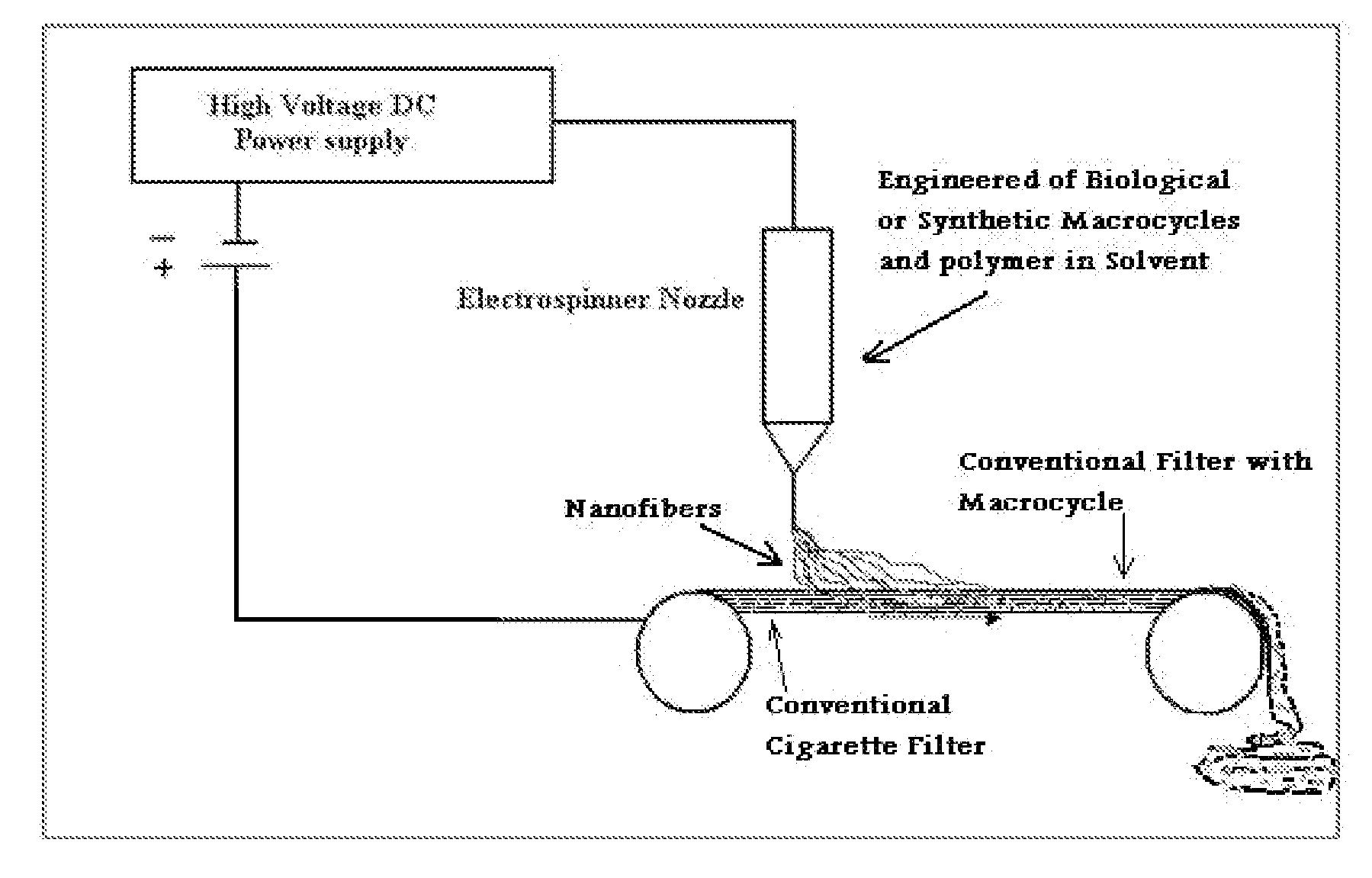
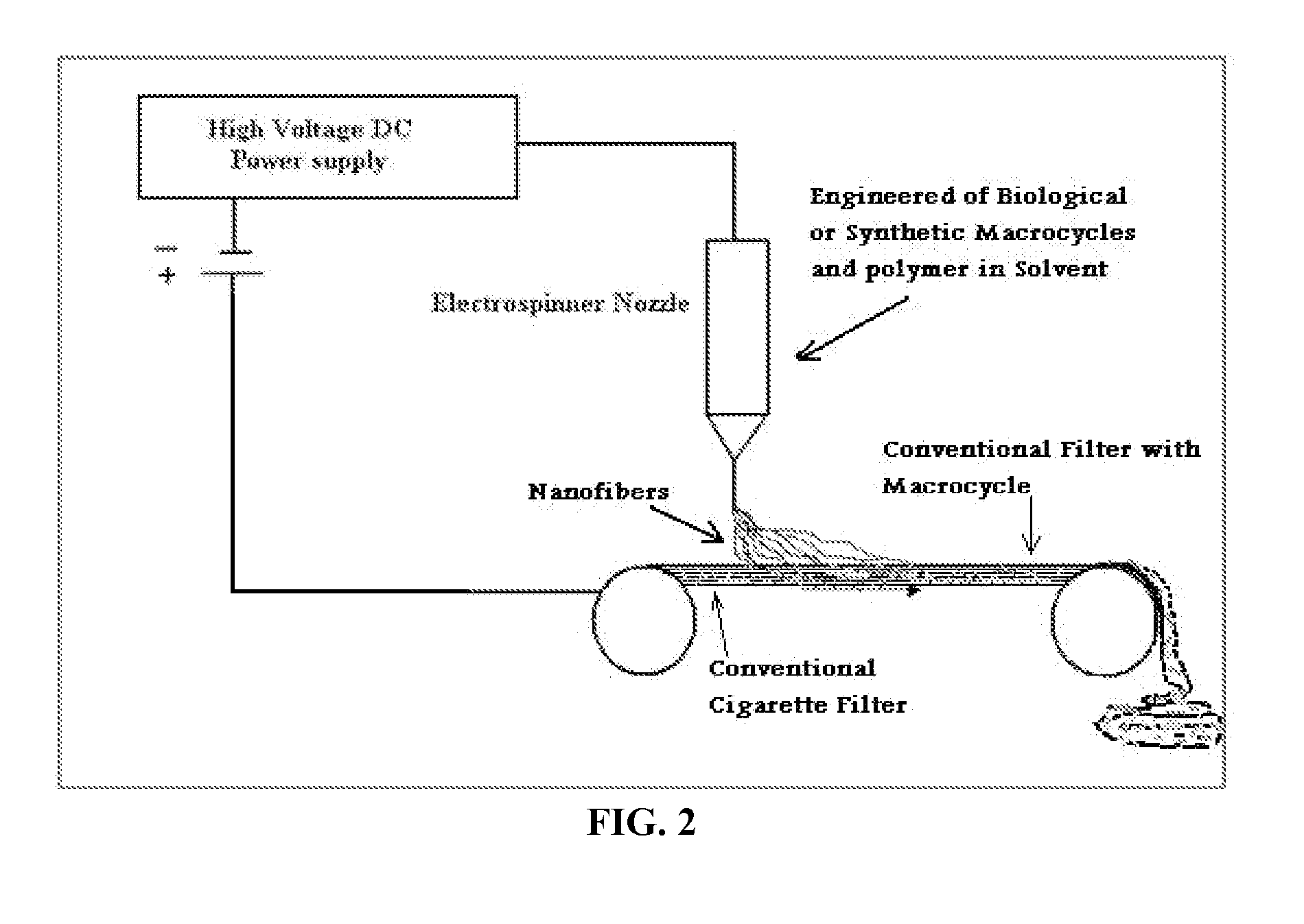
Nanostructural filter for removing toxic compounds
The various embodiments herein provide an electrospun fiber mat filter comprising a cigarette filter containing macrocycle for removing toxic compounds from a toxic material, wherein the toxic material comprises liquid, gas, and cigarette smoke and a method of synthesizing the same. The electrospun fiber mat cigarette filter comprises a biological, organic or synthetic macrocycle, plurality of additives, a solvent and an acceptable polymeric carrier. The biological macromolecules are engineered polyhemoglobin and / or chlorophyll. The biological, organic, or synthetic macrocycle are electrospun with the acceptable polymeric carrier in presence of an abruptly asymmetric electric field to form an electrospun fiber mat. The electrospun fiber mat is made up of networks of plurality of nanofibers.
Owner:GHANAVI JALALEDIN
Method for the preparation of a high-temperature stable oxygen-carrier-containing pharmaceutical composition and the use thereof
ActiveUS7989593B1Increase oxygenationHigh sensitivityPeptide/protein ingredientsMammal material medical ingredientsArteriolar VasoconstrictionWhite blood cell
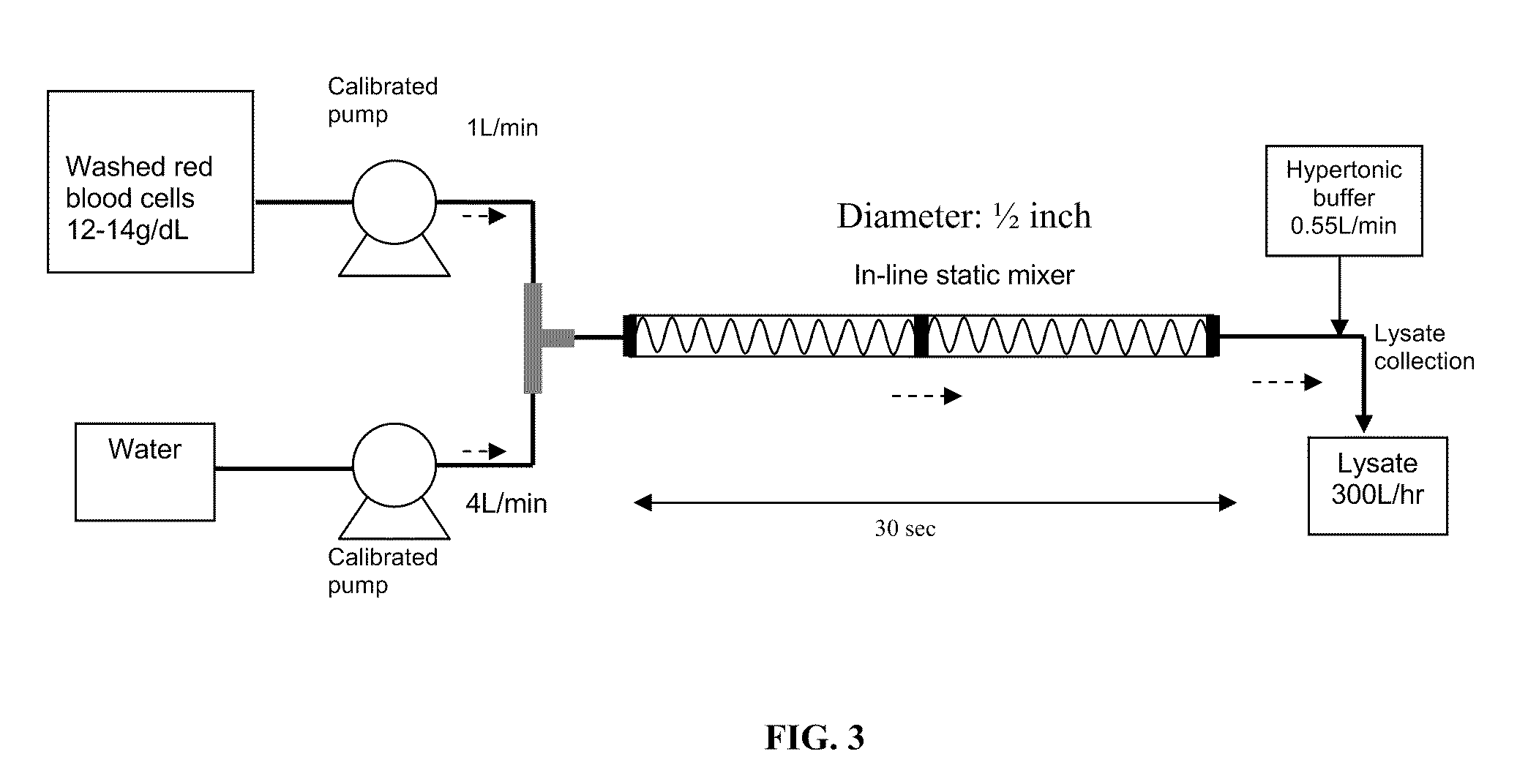
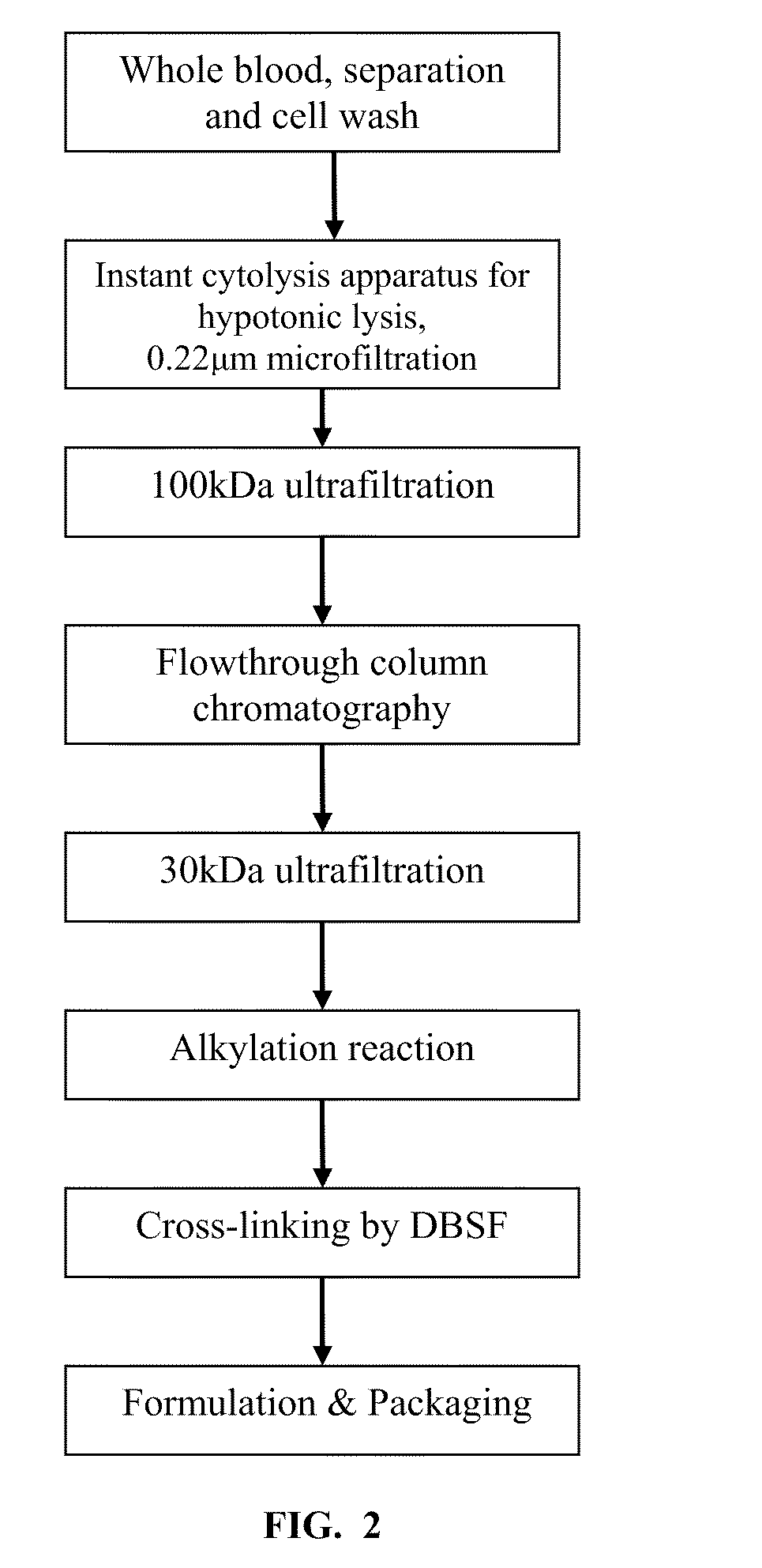
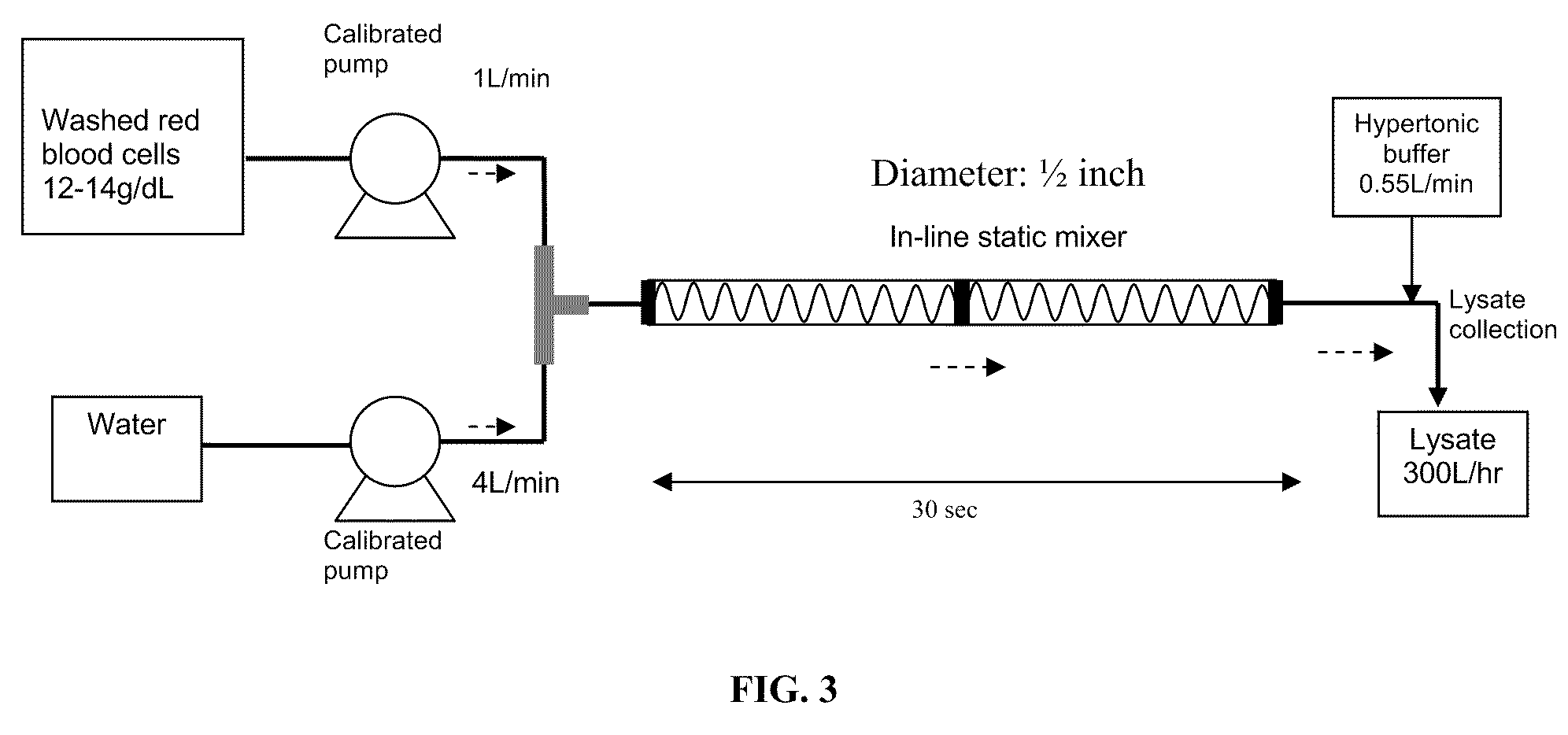
A high temperature-stable and highly purified cross-linked (optionally ≧70% β-β linked) tetrameric hemoglobin with high efficiency of oxygen delivery suitable for use in mammals without causing renal injury and vasoconstriction is provided. The dimeric form of hemoglobin is degenerated and purification processes are performed on red blood cells from whole blood. Controlled hypotonic lysis in an instant cytolysis apparatus prevents lysis of white blood cells. Nucleic acids from white blood cells and phospholipids impurities are not detected. Blocking of reactive sulfhydryl groups by a sulfhydryl reagent is performed in an oxygenated environment. Flowthrough column chromatography removes different plasma protein impurities. N-acetyl cysteine is added to the cross-linked tetrameric hemoglobin to maintain a low level of met-hemoglobin. The stabilized hemoglobin is preserved in an infusion bag with aluminum overwrap to prevent formation of inactive met-hemoglobin from oxygen intrusion. The product finds use in tissue oxygenation and cancer treatment.
Owner:BILLION KING INT
Modified hemoglobin and methods of making same
InactiveUS7501499B2Increase volumeHigh viscosityPeptide/protein ingredientsHaemoglobins/myoglobinsSulfurHemeprotein
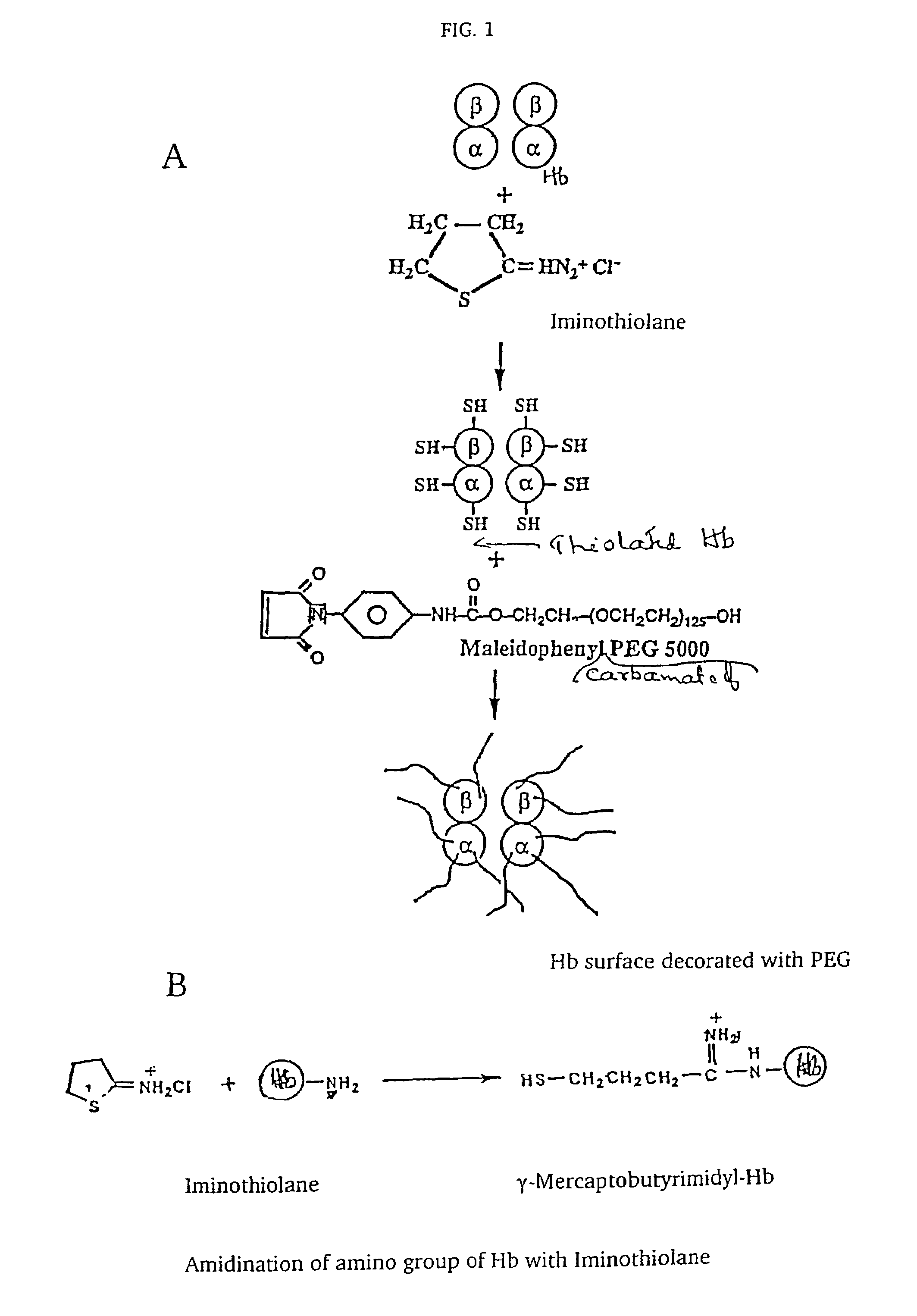
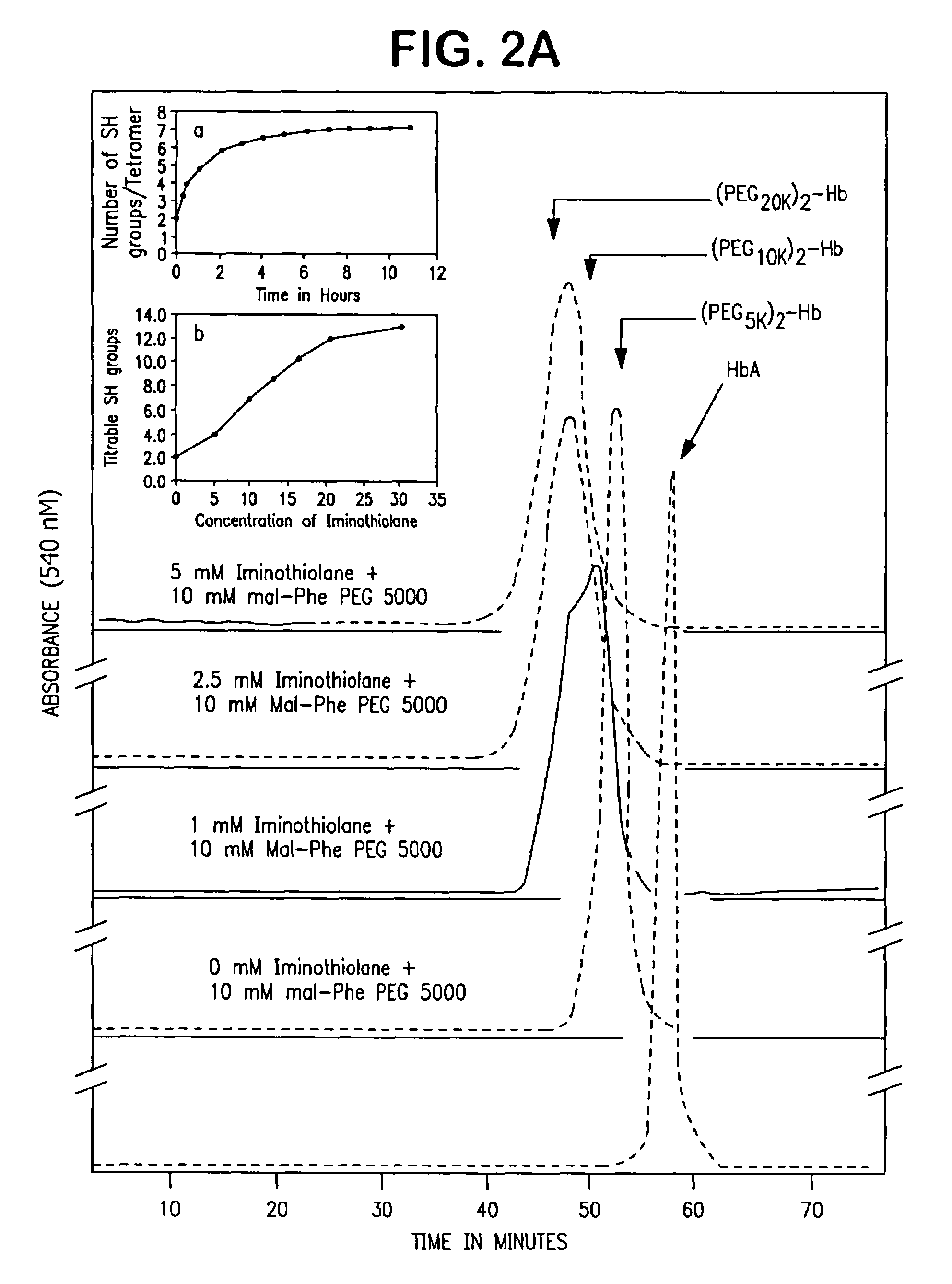
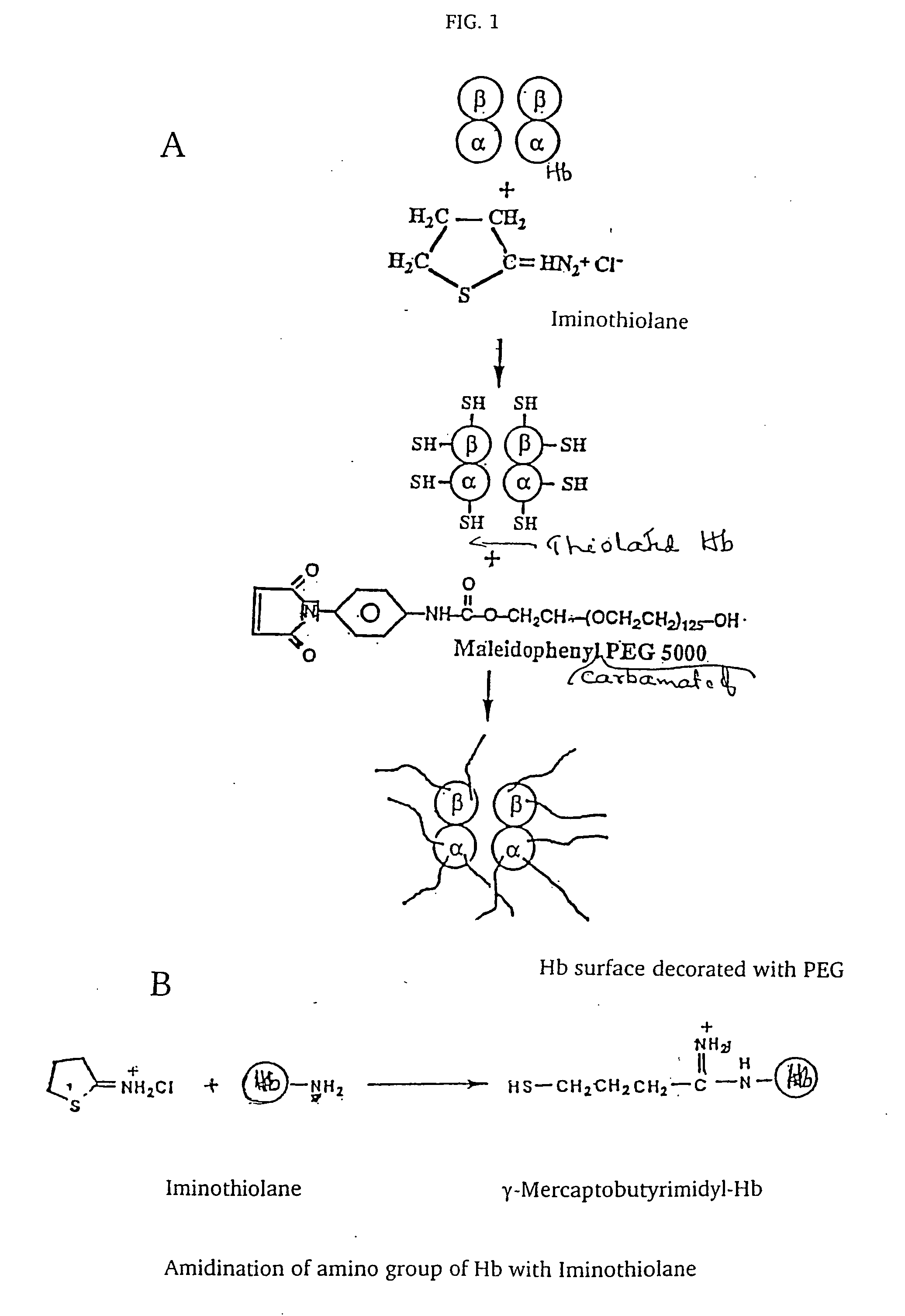
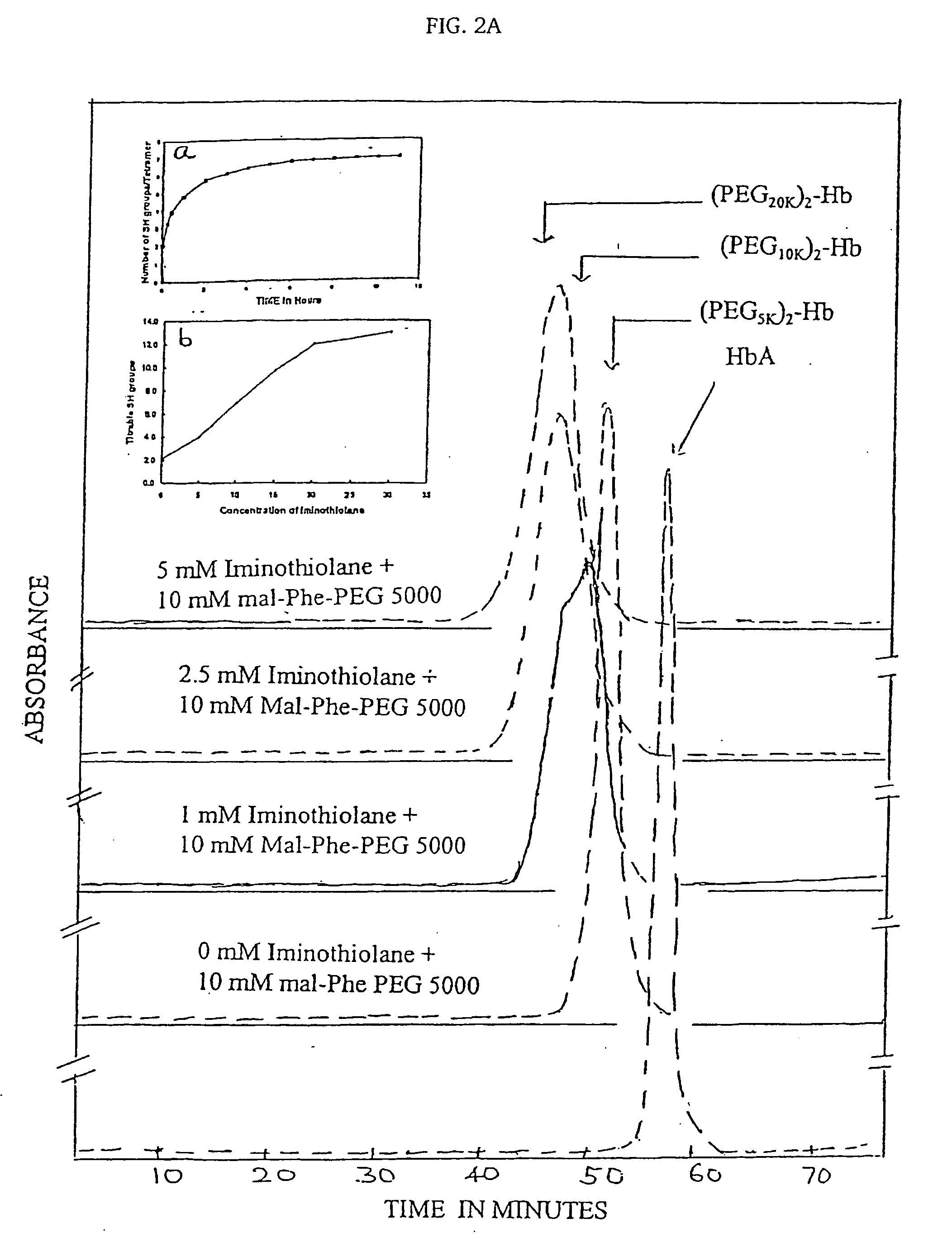
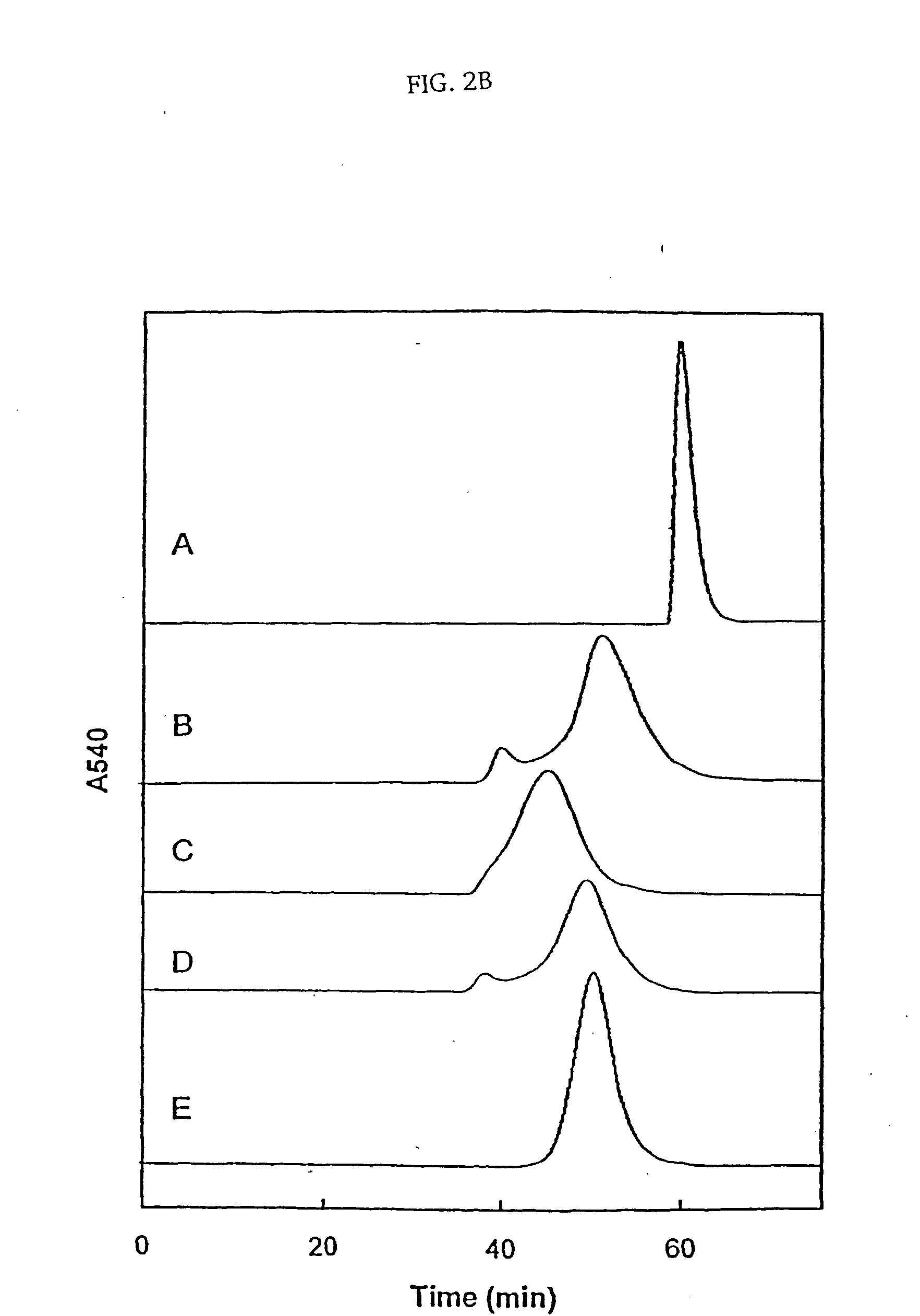
The present invention provides a hemoglobin molecule (Hb) having six ± one PEG chains, wherein two of said PEG chains are bound to Cys-93 (β) of Hb, and the remaining PEG chains are bound to thiol groups introduced on ε-NH2 of Hb. The present invention also provides a process for preparing a modified hemoglobin molecule (Hb), comprising the steps of: (a) reacting Hb with 8-15 fold excess of iminothiolane to form thiolated Hb; and (b) reacting the thiolated Hb with 16-30 fold excess of PEG functionalized with a maleimide moiety, to form the modified Hb.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Personal care composition containing leghemoglobin
A personal care composition comprising leghemoglobin and at least one preservative selected from the group consisting of alcohols, glycols, parabens, hydantoins, quaternary nitrogen-containing compounds, isothiazolinones, aldehyde-releasing agents, and halogenated compounds. Preferably, the leghemoglobin is a nitrogen fixation root nodule extract providing a leghemoglobin concentration in the composition of between 0.0001% and about 10% based upon the total weight of the composition. Also disclosed is a method for preparing the personal care composition.
Owner:ARCH PERSONAL CARE PROD
2-acylaminopropoanol-type glucosylceramide synthase inhibitors
InactiveUS20100256216A1High metabolic stabilityInhibit synthesisBiocideOrganic chemistryStructural formulaGlycolipid
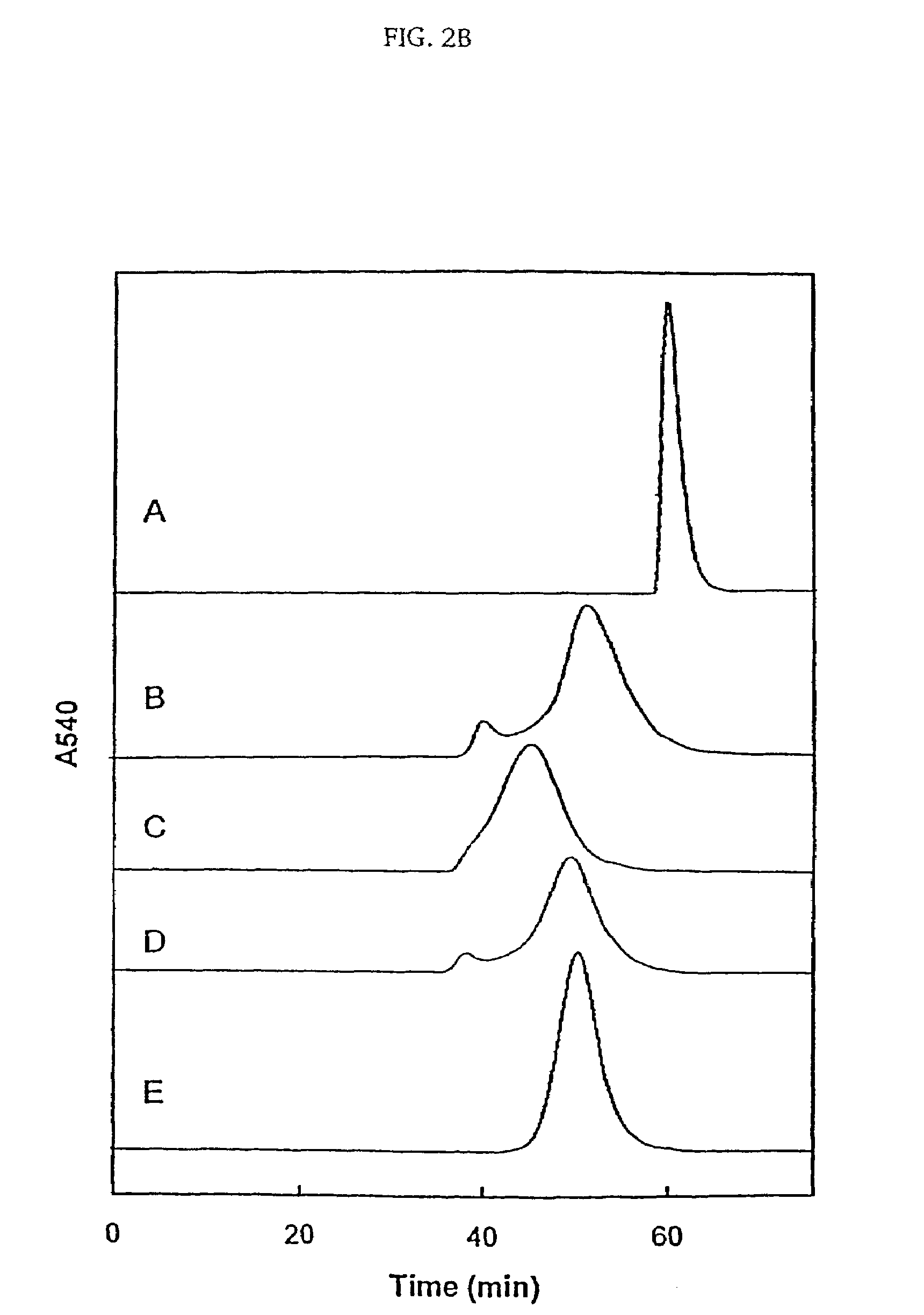
A compound is represented by Structural Formula (I): or a pharmaceutically acceptable salt thereof. A pharmaceutical composition comprises a compound represented by Structural Formula (I) or a pharmaceutically acceptable salt thereof. A method of treating a subject in need thereof comprises administering to the subject a therapeutically effective amount of a compound represented by Structural Formula (I) or a pharmaceutically acceptable salt thereof. The subject has type 2 diabetes; renal hypertrophy or hyperplasia associated with diabetic nephropathy; Tay-Sachs; Gaucher's; or Fabry's disease. Methods of decreasing plasma TNF-α, lowering blood glucose levels, decreasing glycated hemoglobin levels, inhibiting glucosylceramide synthase, and lowering glycosphingolipid concentrations in a subject in need thereof respectively comprise administering to the subject a therapeutically effective amount of a compound represented by Structural Formula (I) or a pharmaceutically acceptable salt thereof.
Owner:GENZYME CORP
Factor viii sequences
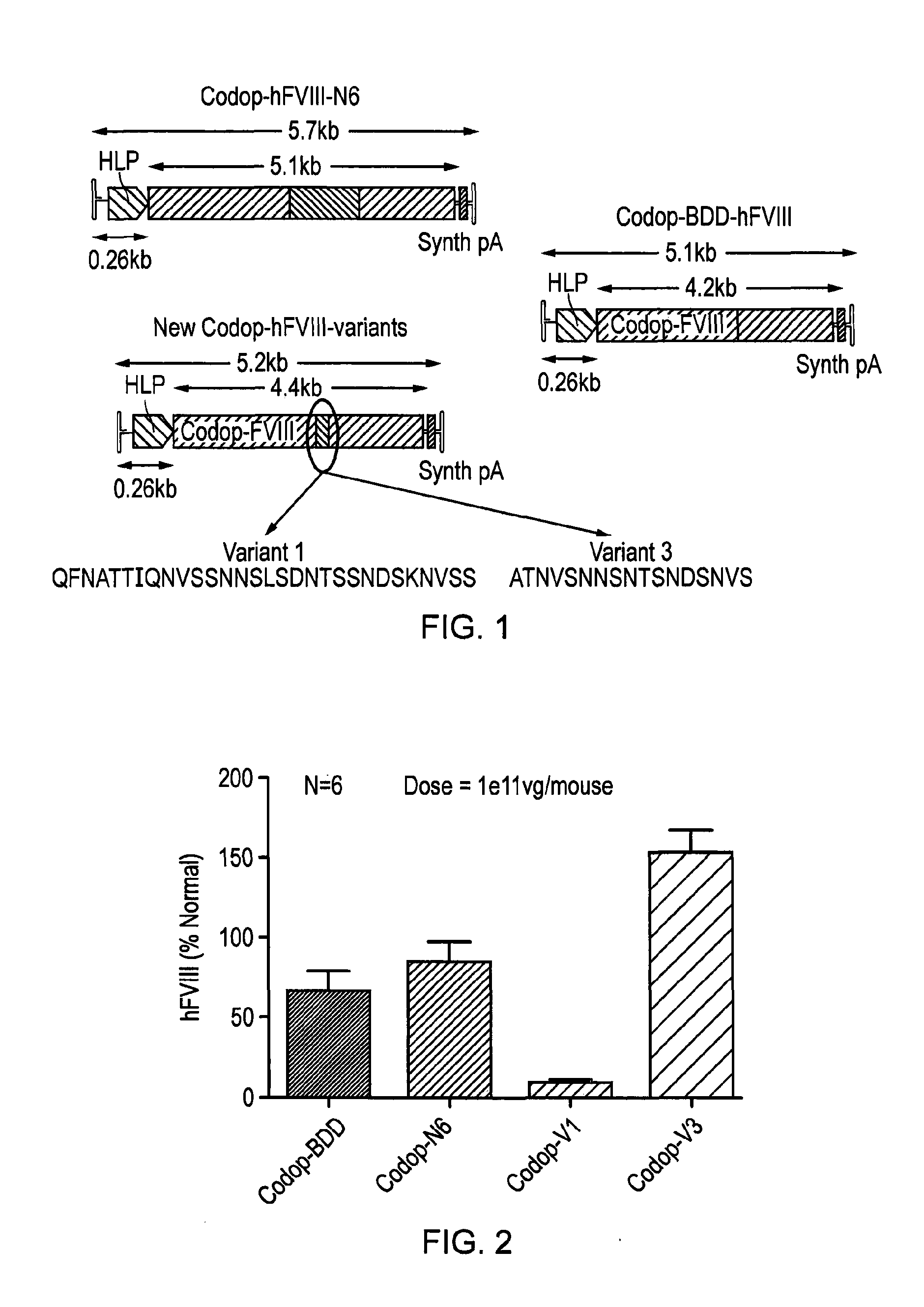
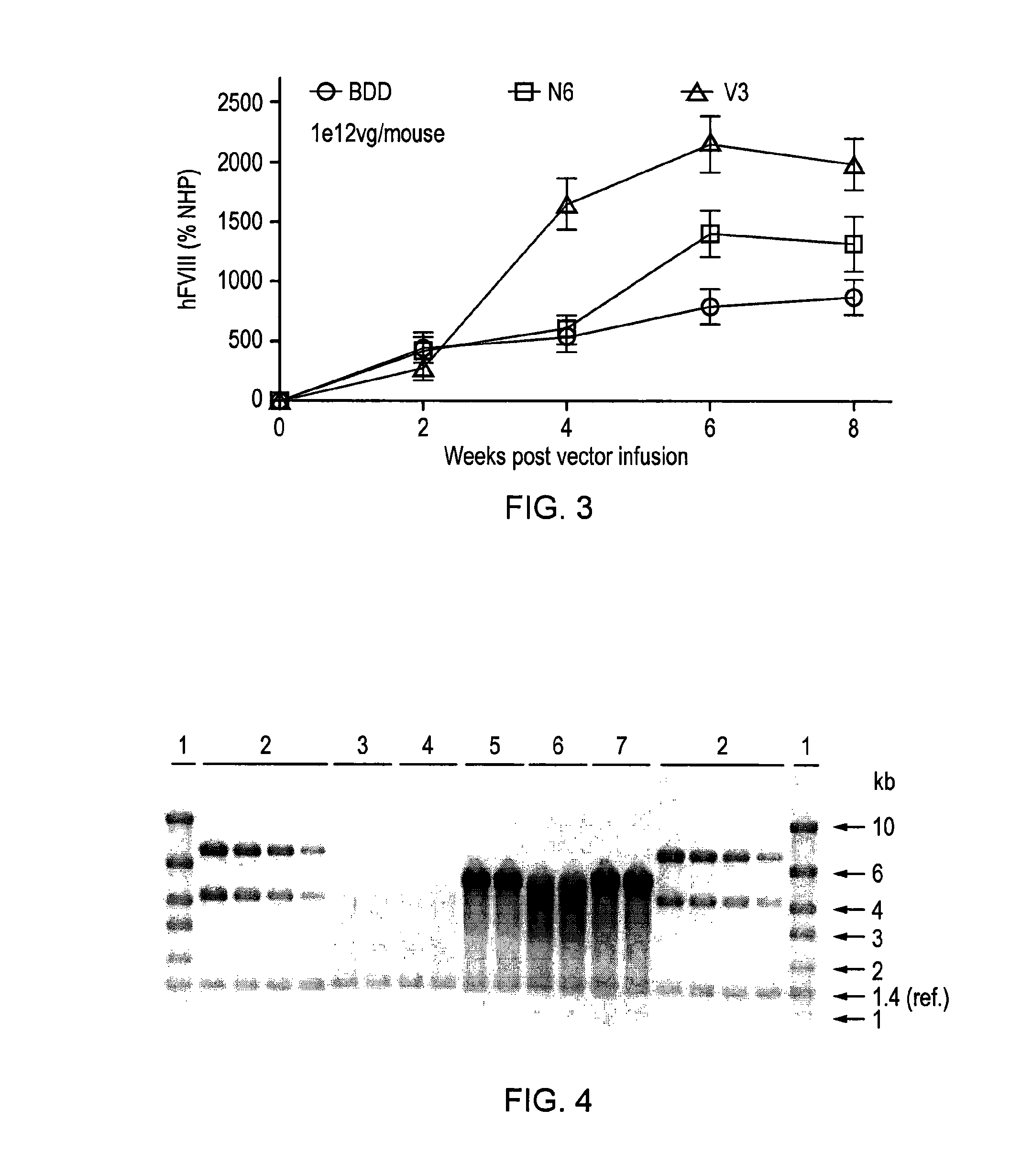
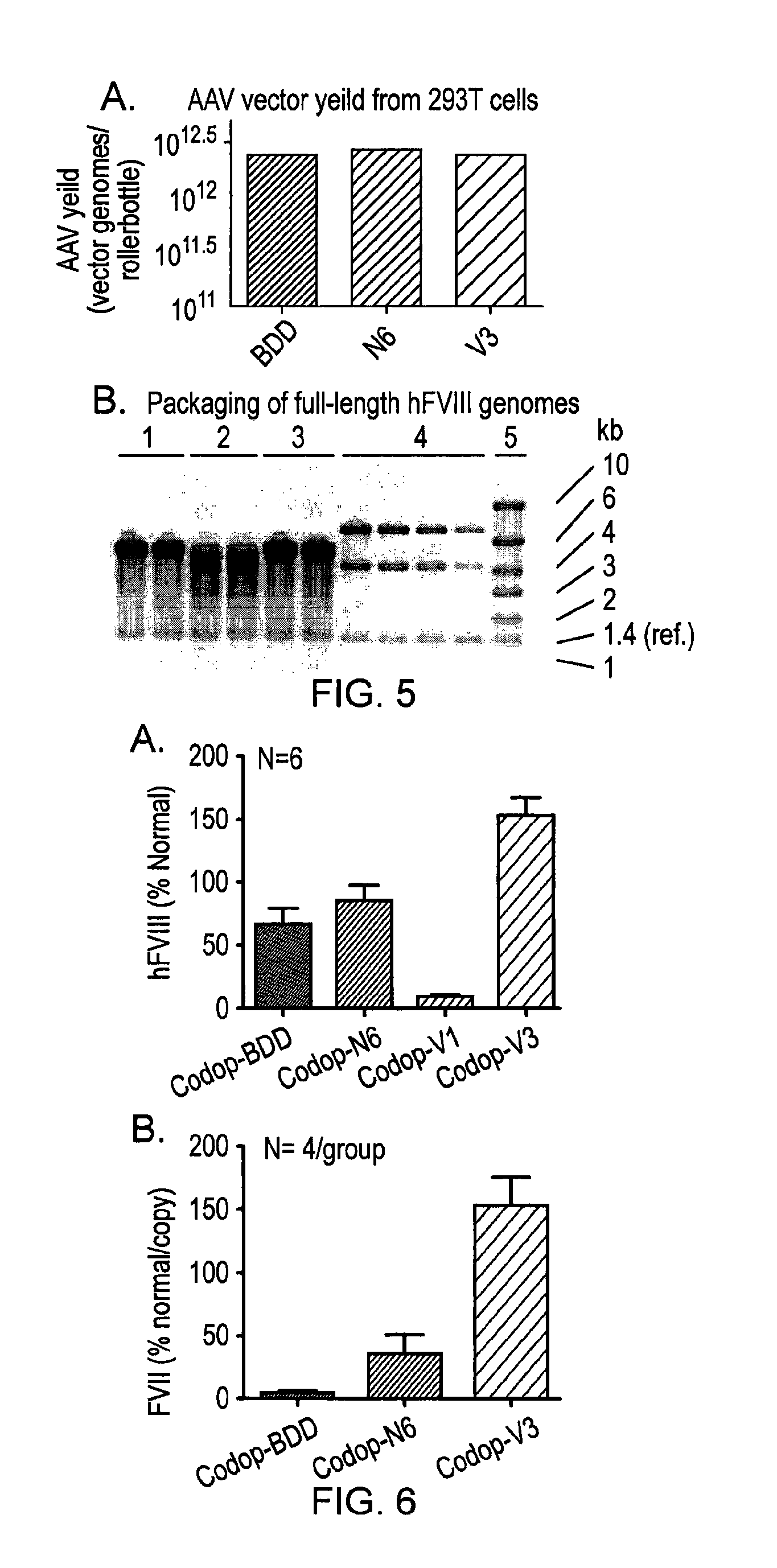
ActiveUS20150158930A1Many symptomIncrease of functional factor VIIIFactor VIIBacteriaGene deliveryNucleotide
There is provided a nucleic acid molecule comprising a nucleotide sequence encoding for a functional factor VIII protein, wherein the portion of the nucleotide sequence encoding for the B domain of the factor VIII protein is between 90 and 111 nucleotides in length and encodes for an amino acid sequence comprising a sequence having at least 85% identity to SEQ ID NO: 4 and which comprises six asparagine residues. Also provided is a functional factor VIII protein, a vector comprising the above nucleic acid molecule, a host cell, a transgenic animal, a method of treating haemophilia, e.g. haemophilia A, and a method for the preparation of a parvoviral gene delivery vector.
Owner:UCL BUSINESS PLC
Modified hemoglobin and methods of making same
InactiveUS20060135753A1Enhanced molecular volumeHigh viscosityPeptide/protein ingredientsHaemoglobins/myoglobinsSulfurHemeprotein
The present invention provides a hemoglobin molecule (Hb) having six±one PEG chains, wherein two of said PEG chains are bound to Cys-93 (β) of Hb, and the remaining PEG chains are bound to thiol groups introduced on ε-NH2 of Hb. The present invention also provides a process for preparing a modified hemoglobin molecule (Hb), comprising the steps of: (a) reacting Hb with 8-15 fold excess of iminothiolane to form thiolated Hb; and (b) reacting the thiolated Hb with 16-30 fold excess of PEG functionalized with a maleimide moiety, to form the modified Hb.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
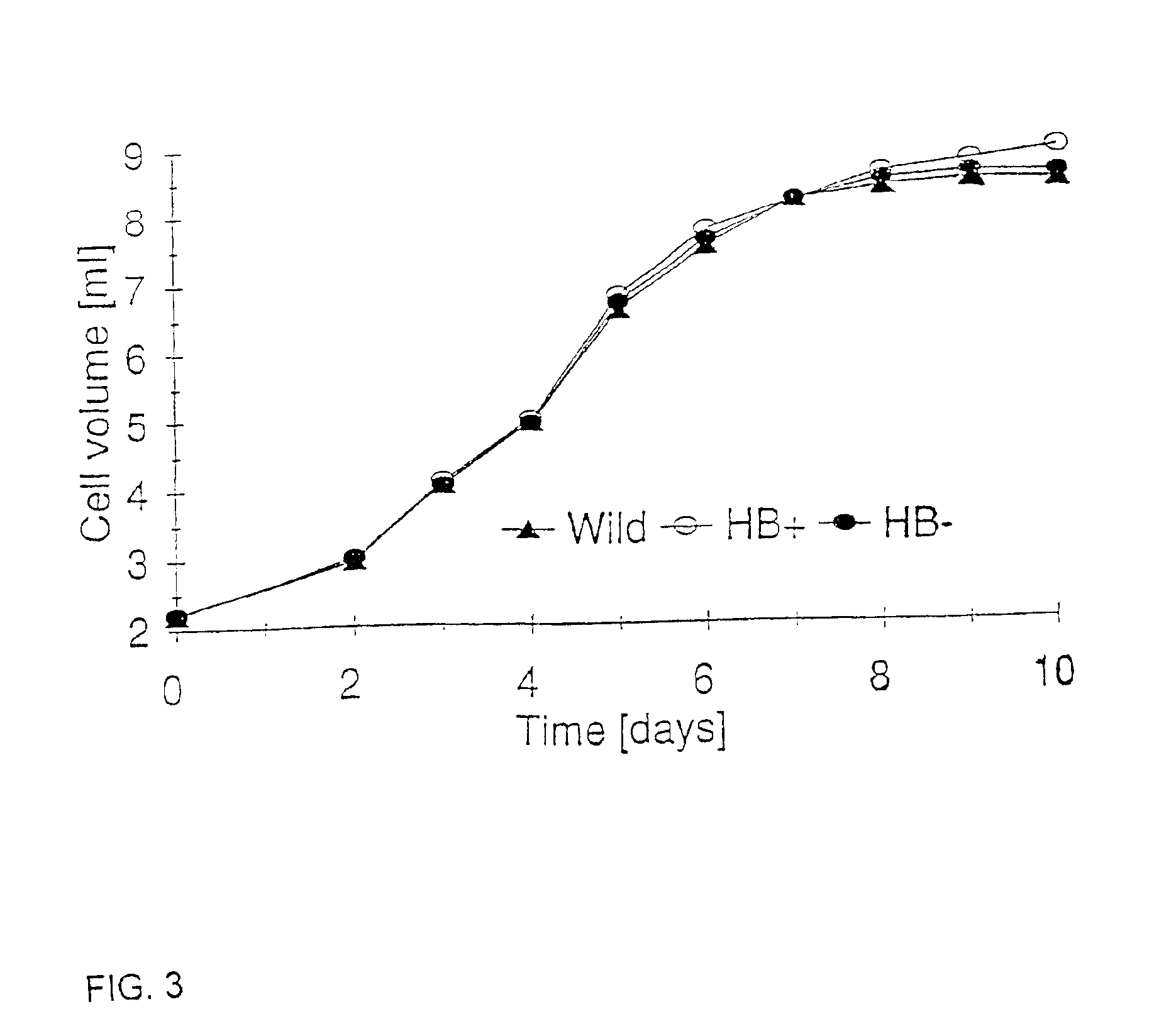
Nonsymbiotic plant hemoglobins to maintain cell energy status
InactiveUS6936749B1Decrease in levelLower Level RequirementsSugar derivativesBacteriaSubstrate-level phosphorylationProgenitor
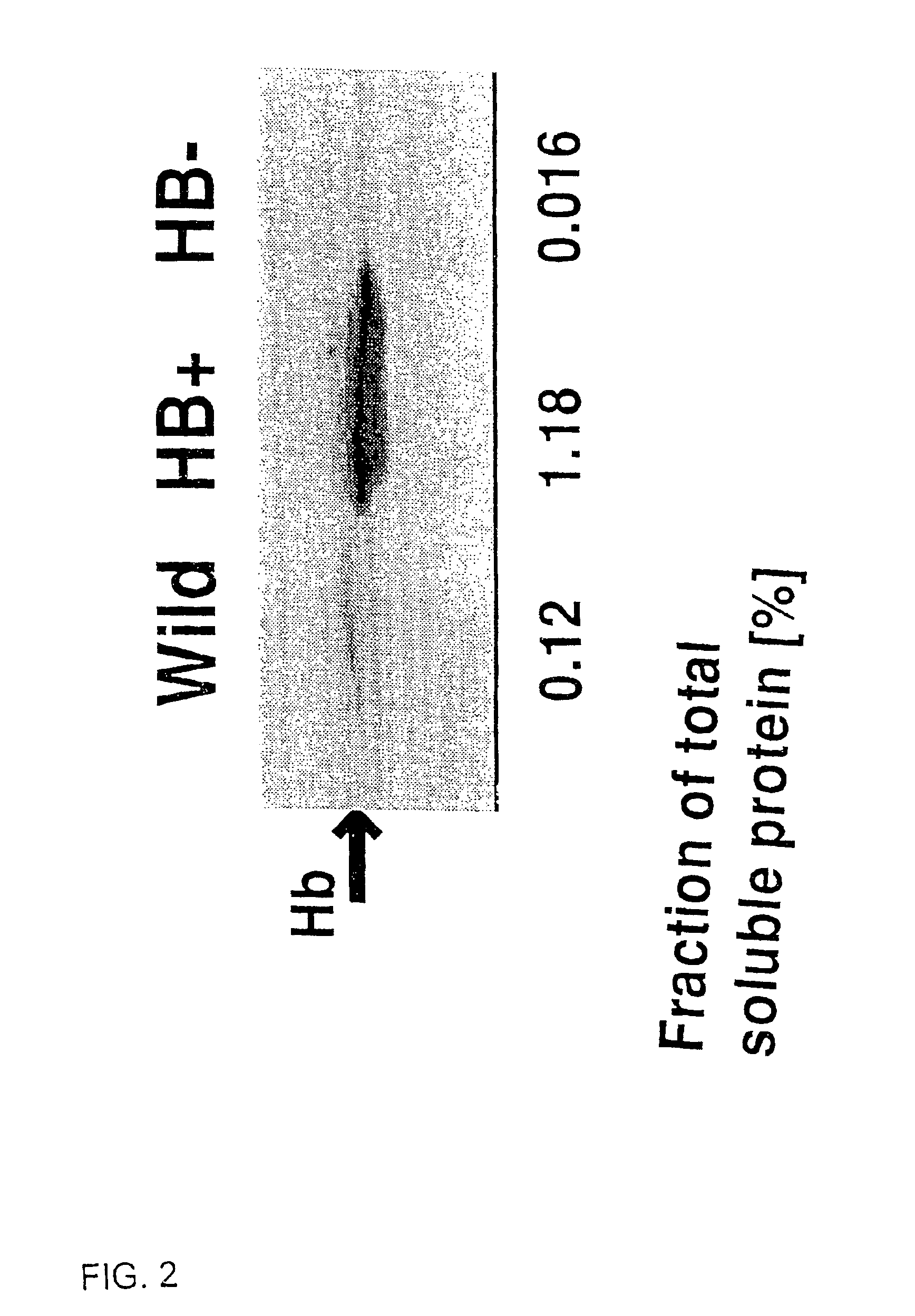
Nonsymbiotic hemoglobins are broadly present across evolution; however, the function of these proteins is unknown. Cultured maize cells have been transformed to constitutively express a barley hemoglobin gene in either the sense (HB+) or antisense (HB−) orientation. Hemoglobin protein in the transformed cell lines was correspondingly higher or lower than in wild type cells under normal atmospheric conditions. Limiting oxygen availability, by placing the cells in a nitrogen atmosphere for 12 hours, had little effect on the energy status of cells constitutively expressing hemoglobin, but had a pronounced effect on both wild type and HB− cells, where ATP levels declined by 27% and 61% respectively. Energy charge was relatively unaffected by the treatment in HB+ and wild type cells, but was reduced from 0.91 to 0.73 in HB− cells suggesting that the latter were incapable of maintaining their energy status under the low oxygen regime. Similar results were observed with P. aeruginosa cells transformed with an Hb expression vector. It is suggested that nonsymbiotic hemoglobins act to maintain the energy status of cells in low oxygen environments and that they accomplish this effect by promoting glycolytic flux through NADH oxidation, resulting in increased substrate level phosphorylation. Nonsymbiotic hemoglobins are likely ancestors of an early form of hemoglobin that sequestered oxygen in low oxygen environments, providing a source of oxygen to oxidize NADH to provide ATP for cell growth and development. This in turn suggests that cells containing increased levels of Hb protein will survive longer under low oxygen tension or high energy demand.
Owner:UNIVERSITY OF MANITOBA
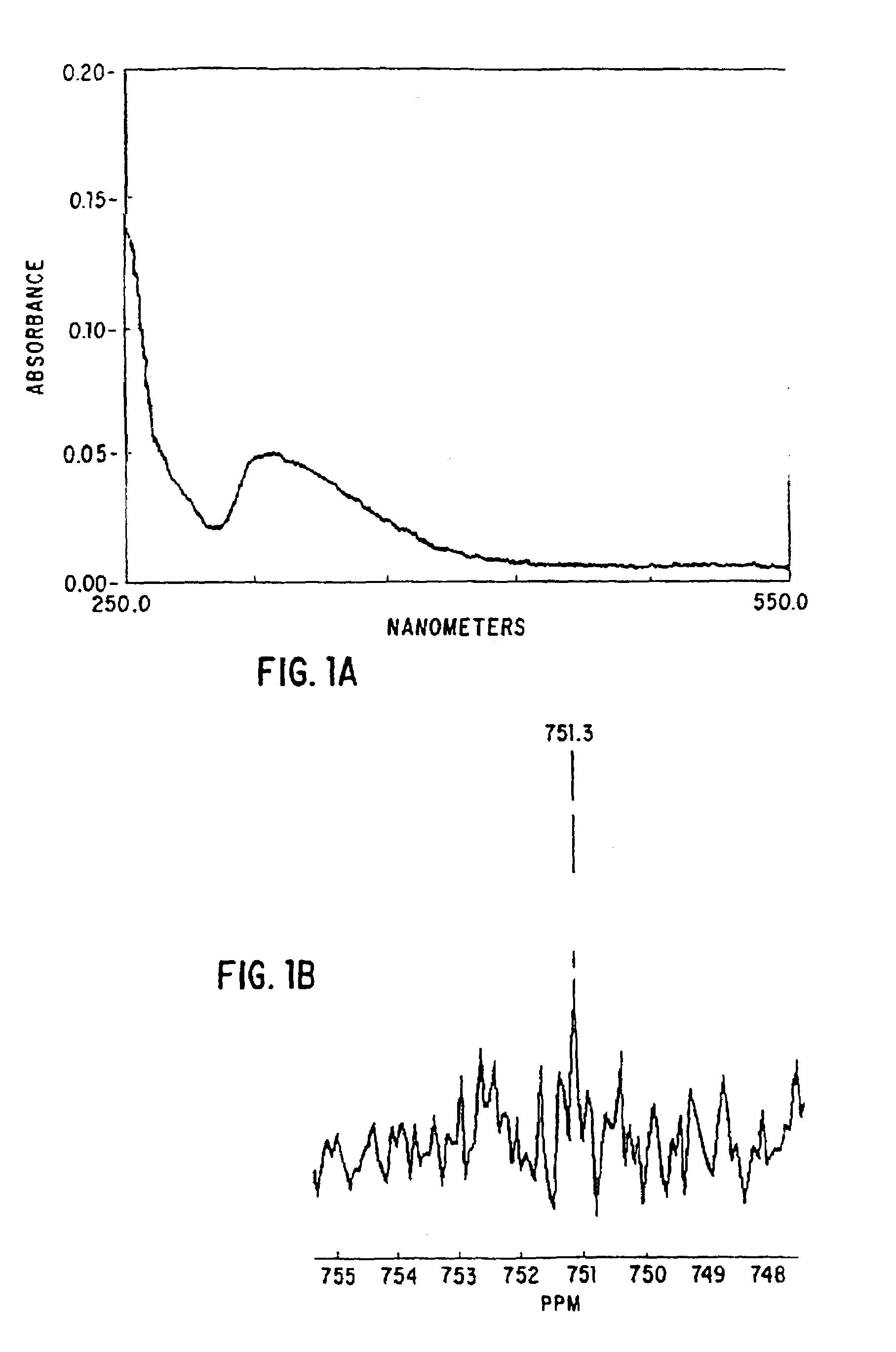
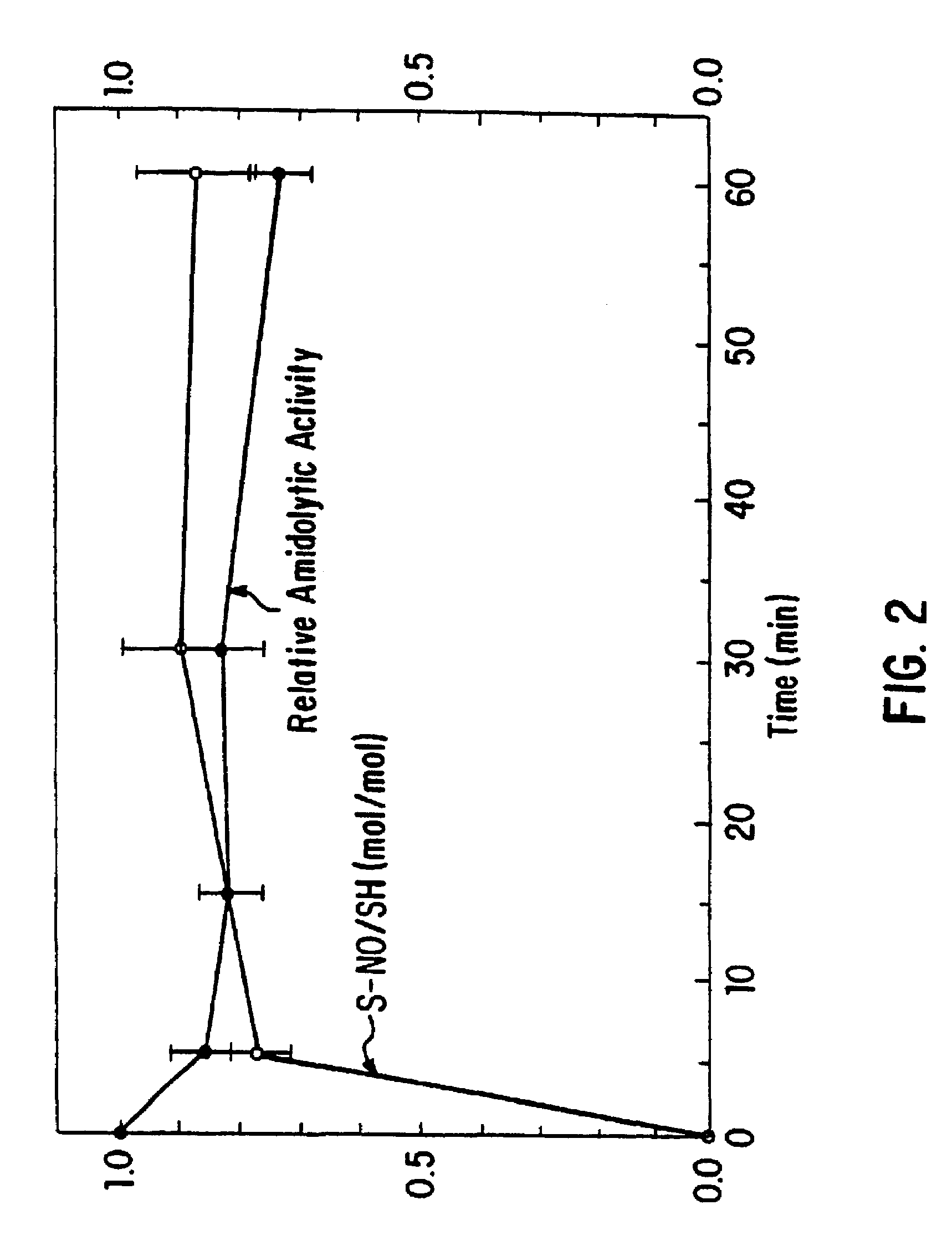
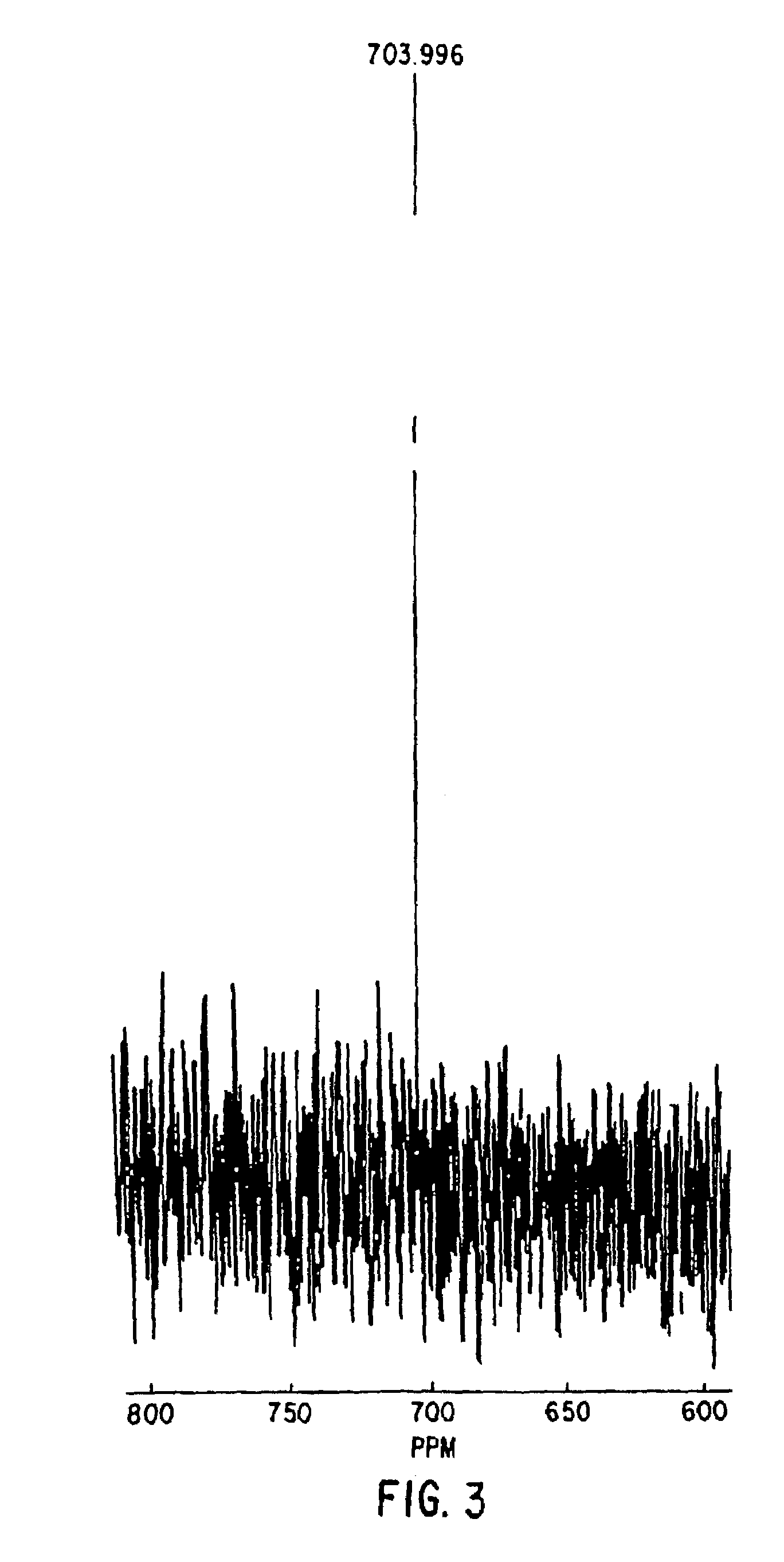
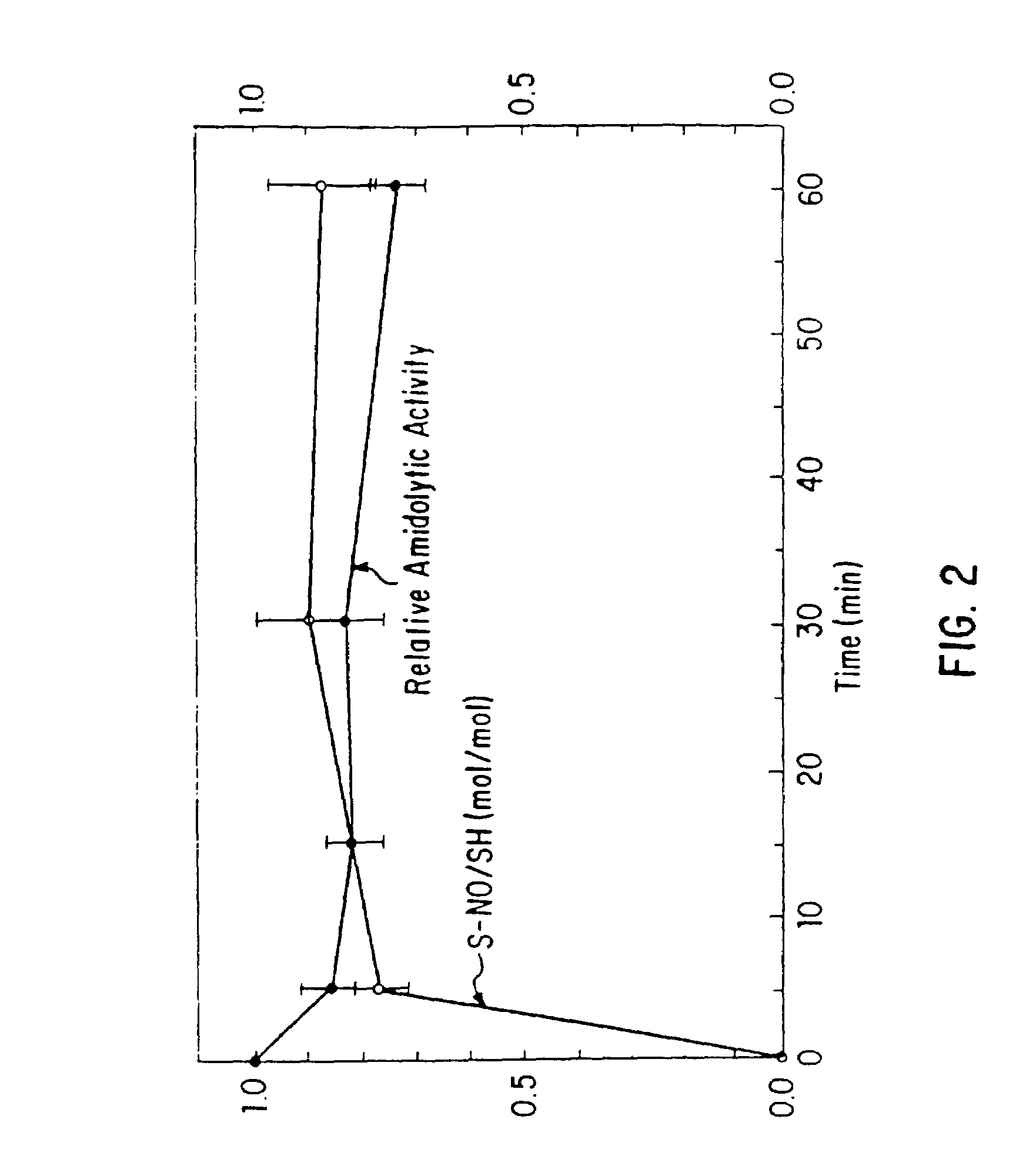
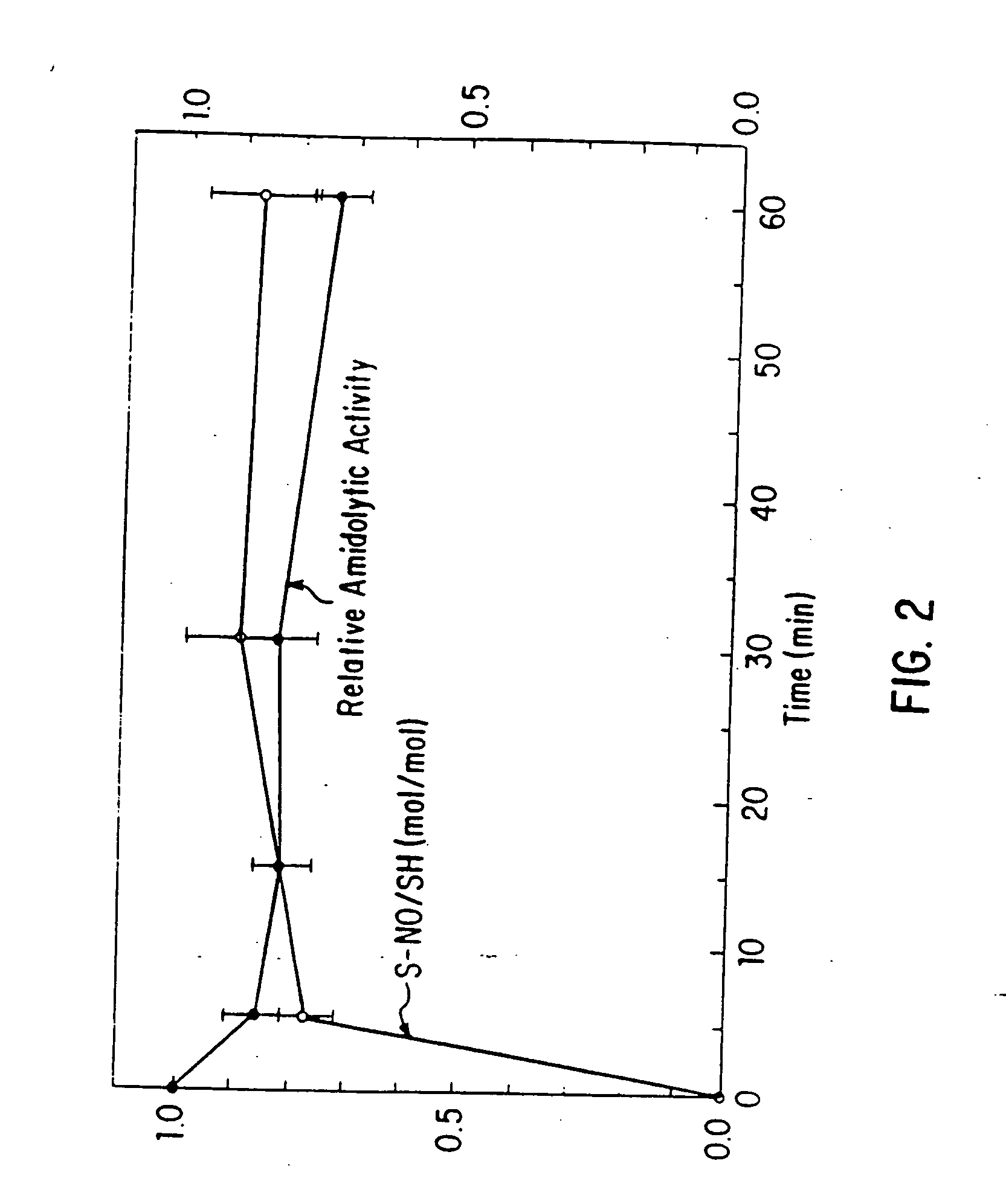
Nitrosated and nitrosylated heme proteins
Nitrosylation of proteins and amino acid groups enables selective regulation of protein function, and also endows the proteins and amino acids with additional smooth muscle relaxant and platelet inhibitory capabilities. Thus, the invention relates to novel compounds achieved by nitrosylation of protein thiols. Such compounds include: S-nitroso-heme proteins, S-nitroso-t-PA, S-nitroso-cathepsin; S-nitroso-lipoproteins; and S-nitroso-immunoglobulins. The invention also relates to therapeutic use if S-nitroso-protein compounds for regulating protein function, cellular metabolism and effecting vasodilation, platelet inhibition, relaxation of non-vascular smooth muscle, and increasing blood oxygen transport by hemoglobin and myoglobin. The compounds are also used to deliver nitric oxide in its most bioactive form in order to achieve the effects described above, or for in vitro nitrosylation of molecules present in the body. The invention also relates to the nitrosylation of oxygen, carbon and nitrogen moieties present on proteins and amino acids, and the use thereof to achieve the above physiological effects.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Use Of Dipyridamole For Treatment Of Resistance To Platelet Inhibitors
InactiveUS20090048173A1Reduce decreaseBiocidePeptide/protein ingredientsDipyridamolePlatelet inhibitor
Owner:EISERT WOLFGANG +1
Personal care composition containing leghemoglobin
Owner:GRUBER JAMES V
Inhibitors of hemeprotein-catalyzed lipid peroxidation
Methods and compounds for the treatment or prevention of oxidative damage in a mammalian subject. The treatment and / or prevention may be on inhibiting heme-induced lipid peroxidation. Also discloses are methods and compounds for treating or preventing isoprostane-mediated tissue damage.
Owner:VANDERBILT UNIV
Nitric oxide-blocked cross-linked tetrameric hemoglobin
InactiveUS7504377B2Increase capacityImprove abilitiesBiocidePeptide/protein ingredientsCross-linkThiol
The present invention includes compositions containing carboxamidomethylated cross-linked hemoglobin where the cysteine moiety of the hemoglobin includes a thiol protecting group and where the hemoglobin has a reduced ability to bind with nitric oxide. Preferably, the hemoglobin is deoxygenated, endotoxin free, and stroma free. The present invention also includes method of preparation, process of preparation and the method of use including supplementing blood volume in mammals and treating disorders in mammals where oxygen delivery agents are of benefit.
Owner:IKOR +1
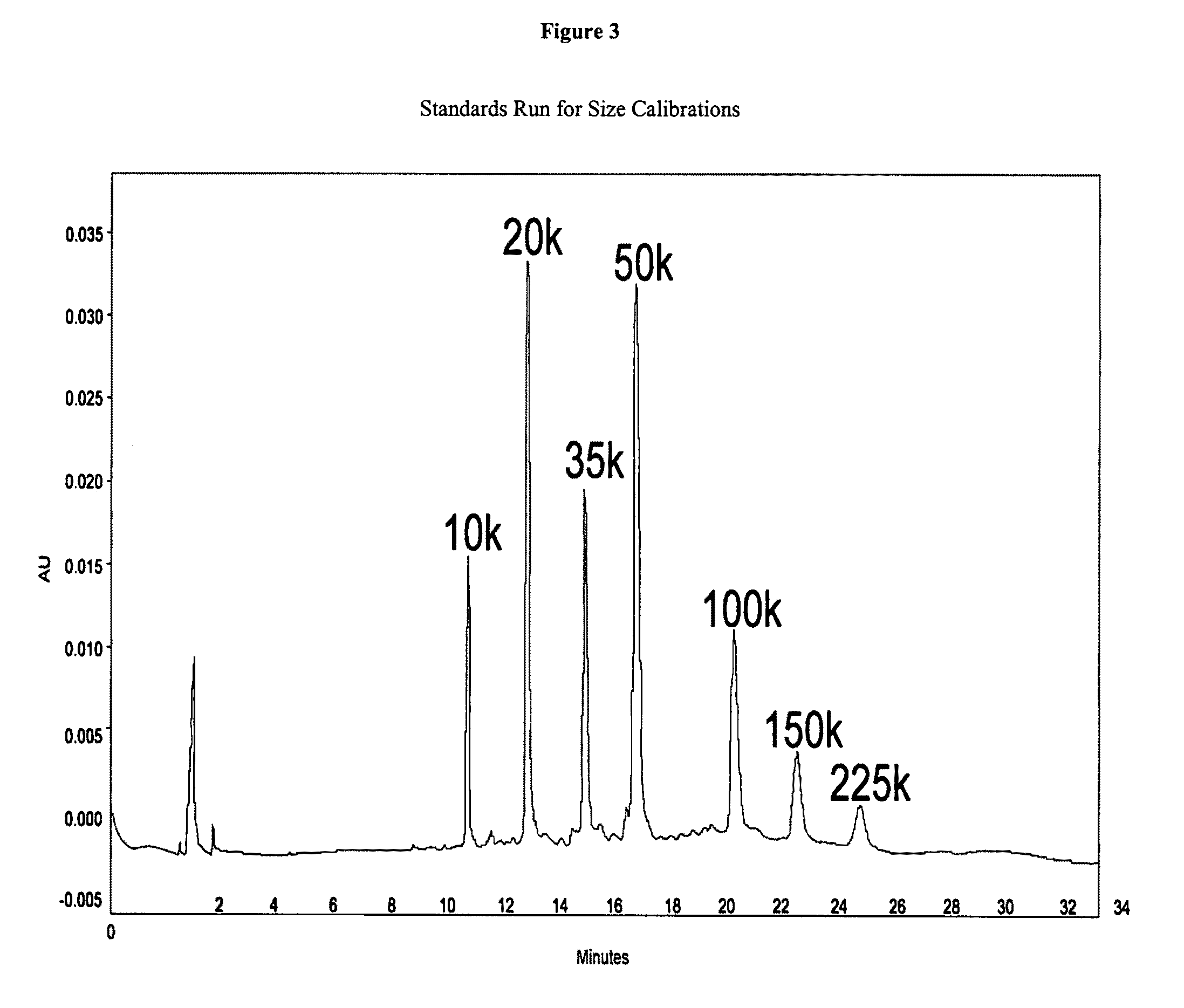
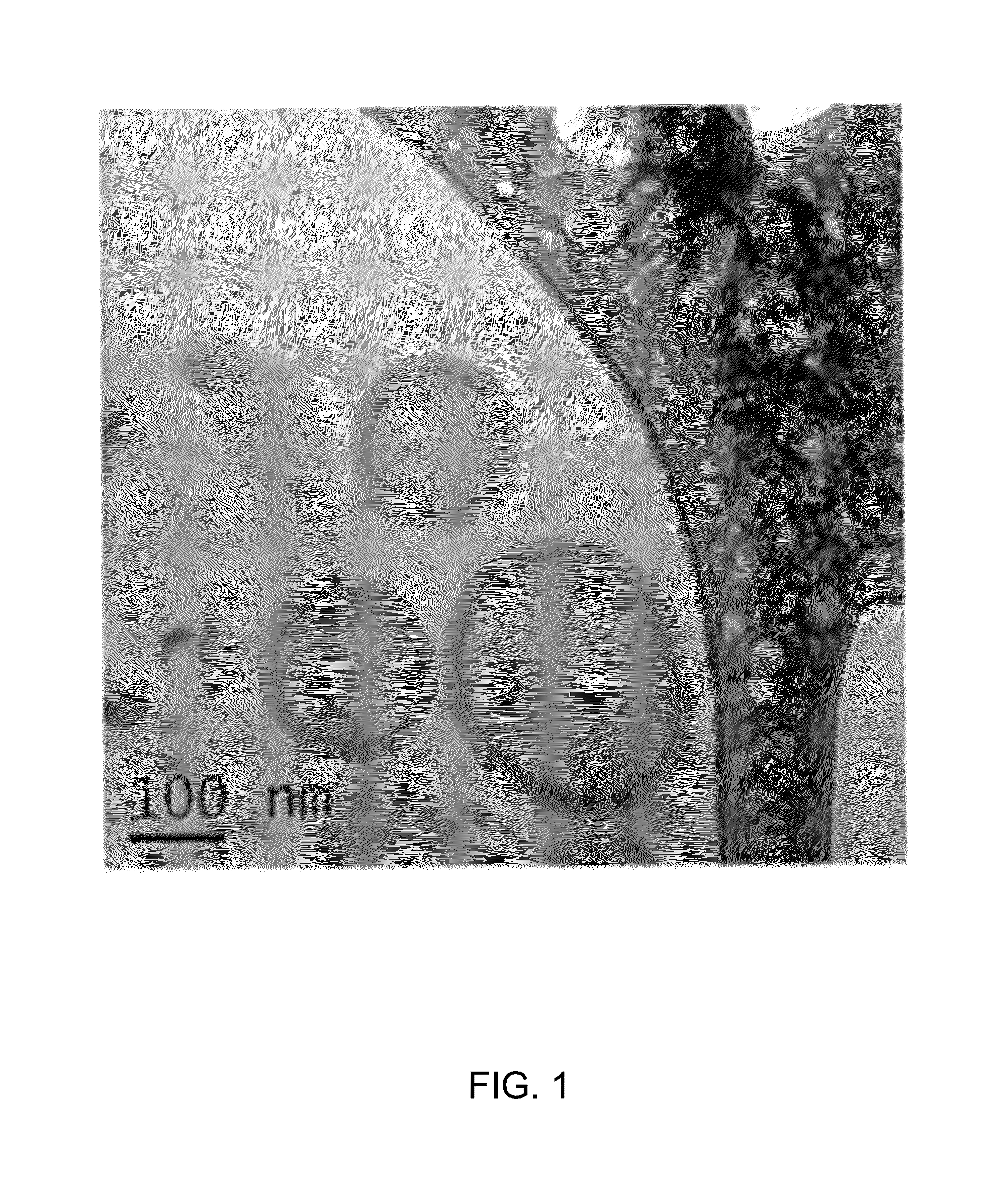
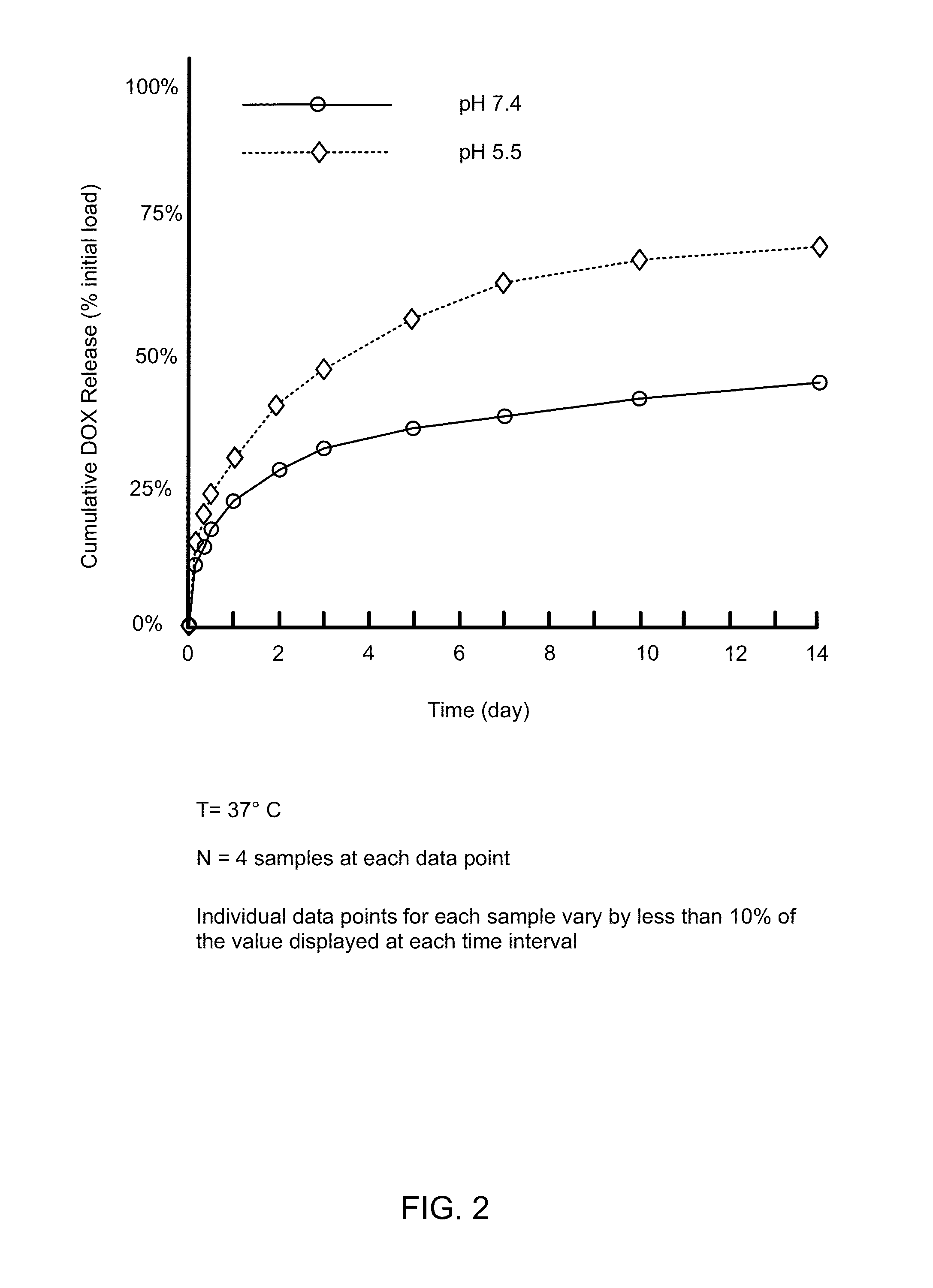
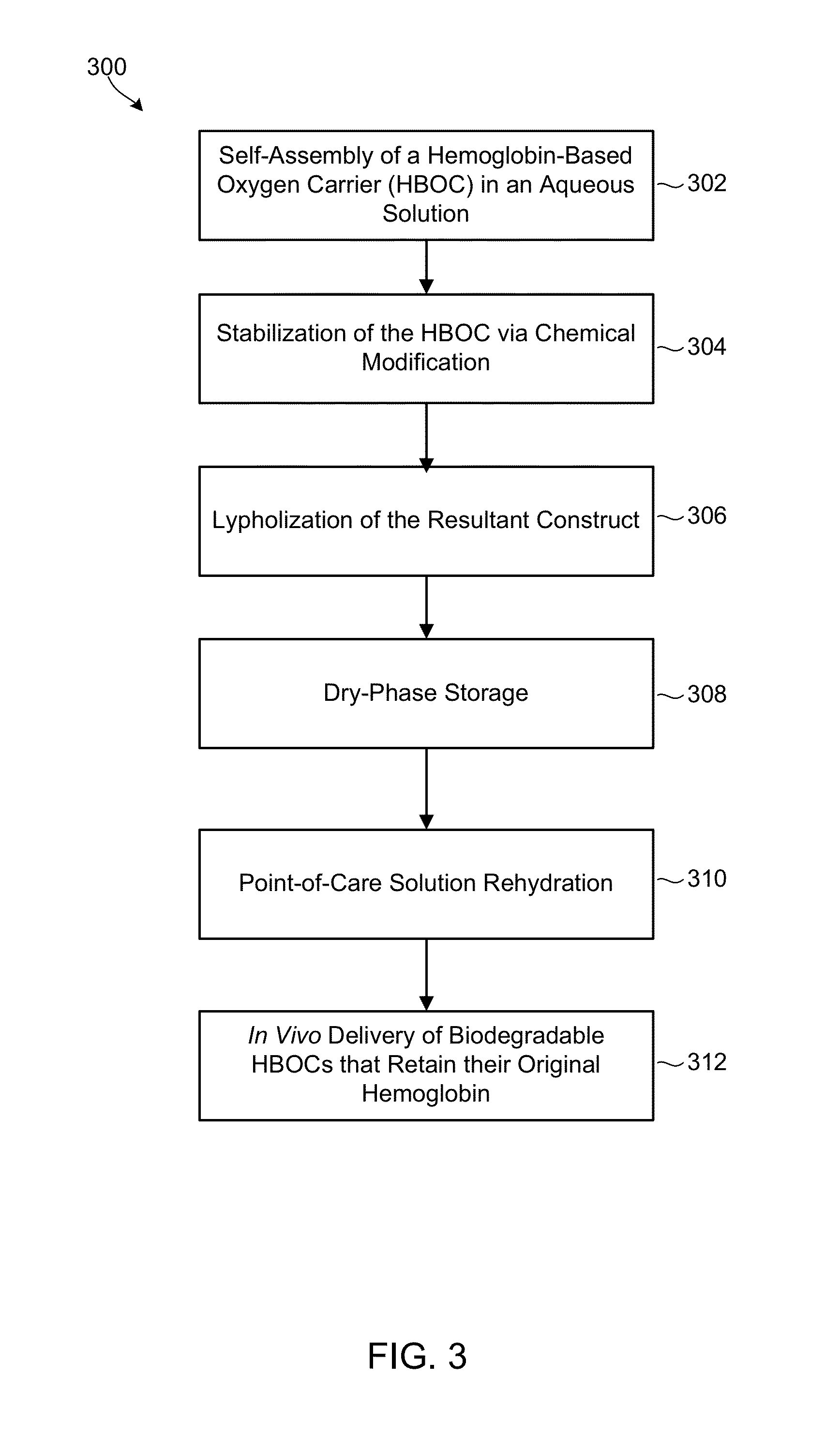
Biodegradable nanoparticles as novel hemoglobin-based oxygen carriers and methods of using the same
ActiveUS8808748B2Low oxygenation of tissueLow oxygenationPowder deliveryPeptide/protein ingredientsEthylene oxideHemeprotein
Compositions of matter and methods for making, storing and administering artificial blood substitutes. Artificial blood substitutes may have oxygen carriers that encapsulate an oxygen-binding compound in a polymer vesicle. Oxygen-binding compounds may include hemoglobin, myoglobin, or other oxygen binding compounds having characteristics similar to hemoglobin. Oxygen carriers may include nanoparticles, polymers and / or polymersomes comprising of poly(ethylene oxide)-block-poly(ε-caprolactone) (PEO-b-PCL) and related diblock copolymers of poly(ethylene oxide)-block-poly(γ-methyl ε-caprolactone) (PEO-b-PMCL). The oxygen carriers may have tunable oxygen-binding capacities, uniform and appropriately small size distributions, and human bloodlike viscosities and oncotic properties.
Owner:POSEIDA THERAPEUTICS INC
Electrochemical biosensor electrode for detection of hydrogen peroxide and preparation method thereof
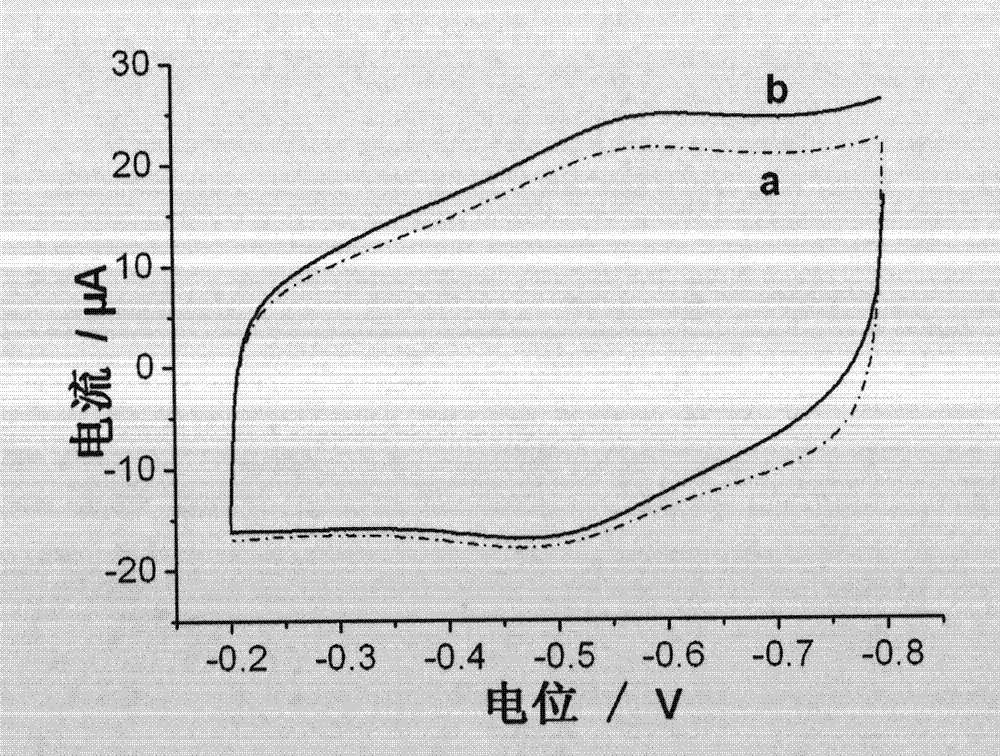
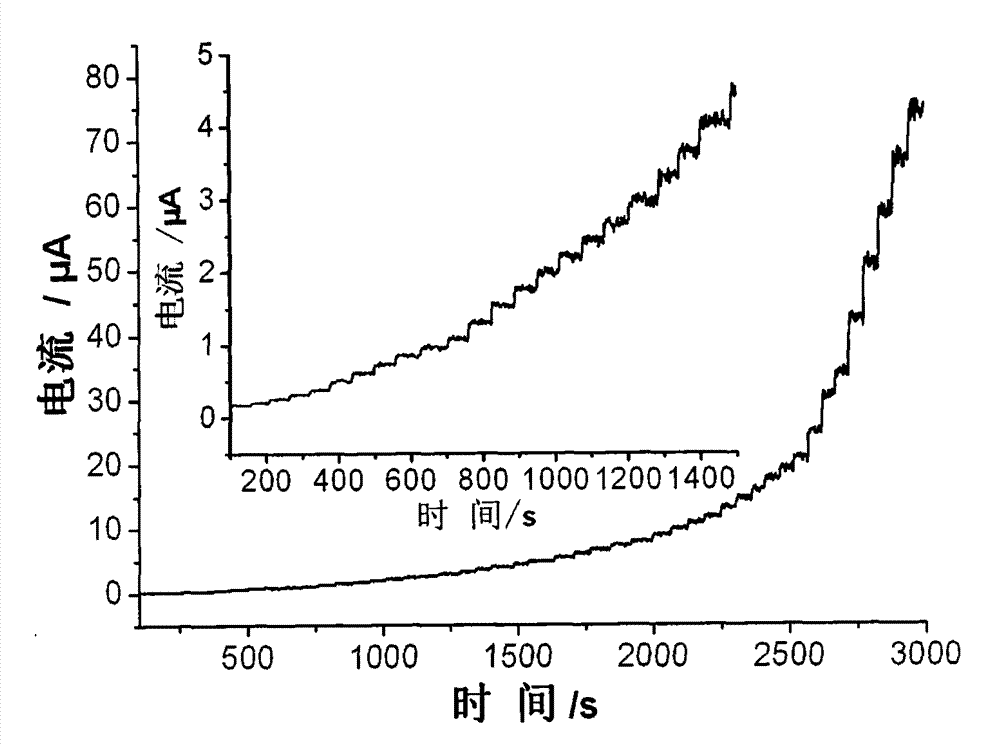
InactiveCN103207224AFacilitates electron transferIncreased sensitivityMaterial analysis by electric/magnetic meansFiberElectrochemical biosensor
The invention provides an enzyme biosensor for detection of hydrogen peroxide and a preparation method thereof. The biosensor employs a classic three-electrode system, wherein a reference electrode is a saturated calomel electrode, a counter electrode is a platinum electrode and a working electrode is a glassy carbon electrode. The biosensor provided by the invention promotes electron transfer between hemoglobin and the electrodes mainly by using a composite material of a helical carbon nanofiber and a colloidal gold nanoparticle as a carrier of hemoglobin, so sensitivity of detection of hydrogen peroxide is effectively improved and a satisfactory result is achieved.
Owner:CHANGSHU INSTITUTE OF TECHNOLOGY
Nitrosylation of protein SH groups and amino acid residues as a therapeutic modality
InactiveUS7157500B2Organic active ingredientsHydrolasesVascular smooth muscle relaxationPlatelet inhibition
Nitrosylation of proteins and amino acid groups enables selective regulation of protein function, and also endows the proteins and amino acids with additional smooth muscle relaxant and platelet inhibitory capabilities. Thus, the invention relates to novel compounds achieved by nitrosylation of protein thiols. Such compounds include: S-nitroso-t-PA, S-nitroso-cathepsin; S-nitroso-lipoprotein; and S-nitroso-immunoglobulin. The invention also relates to therapeutic use of S-nitroso-protein compounds for regulating protein function, cellular metabolism and effecting vasodilation, platelet inhibition, relaxation of non-vascular smooth muscle, and increasing blood oxygen transport by hemoglobin and myoglobin. The compounds are also used to deliver nitric oxide in its most bioactive form in order to achieve the effects described above, or for in vitro nitrosylation of molecules present in the body. The invention also relates to the nitrosylation of oxygen, carbon and nitrogen moieties present on proteins and amino acids, and the use thereof to achieve the above physiological effects.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Cyanideless hemolysin and its use
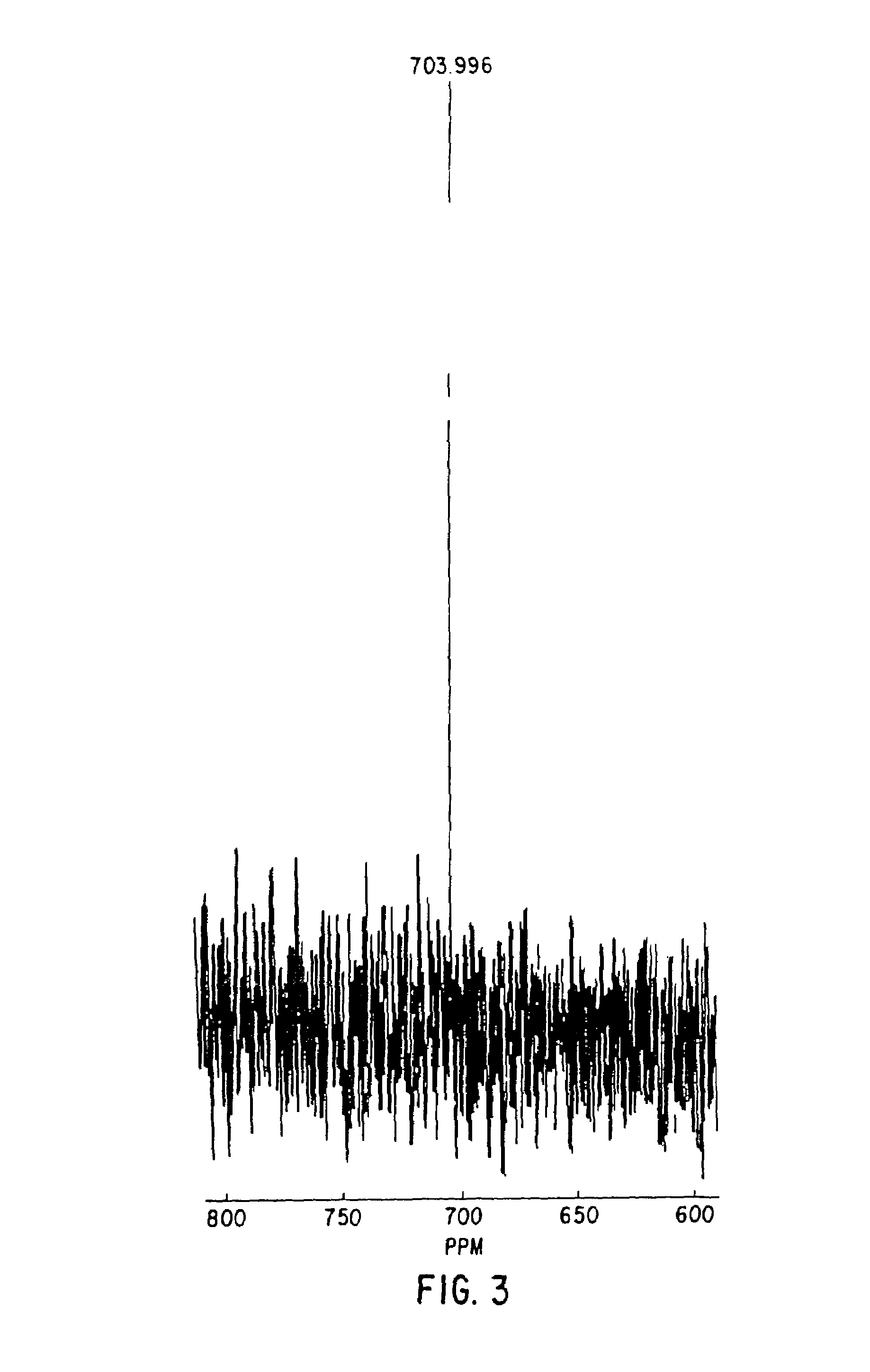
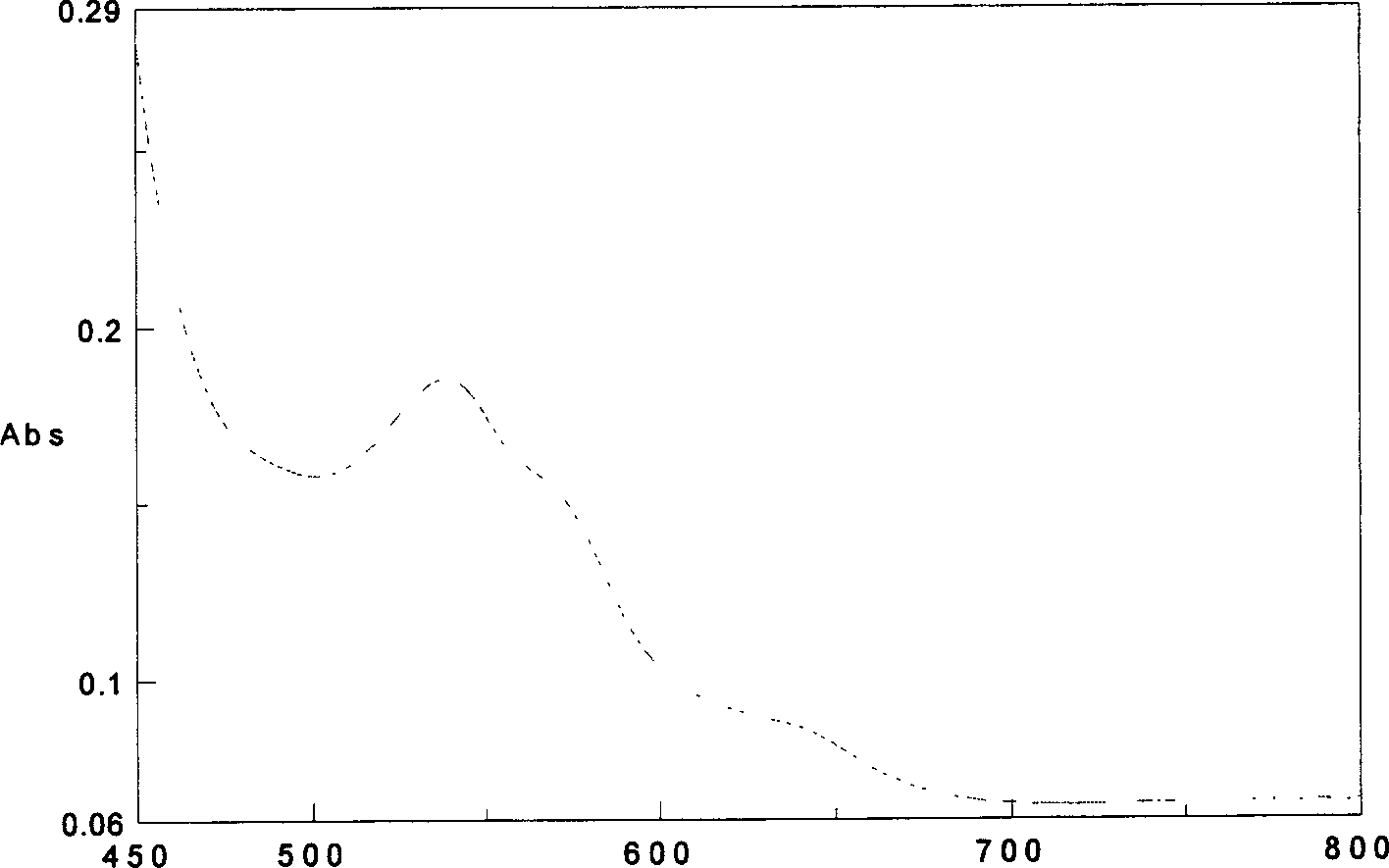
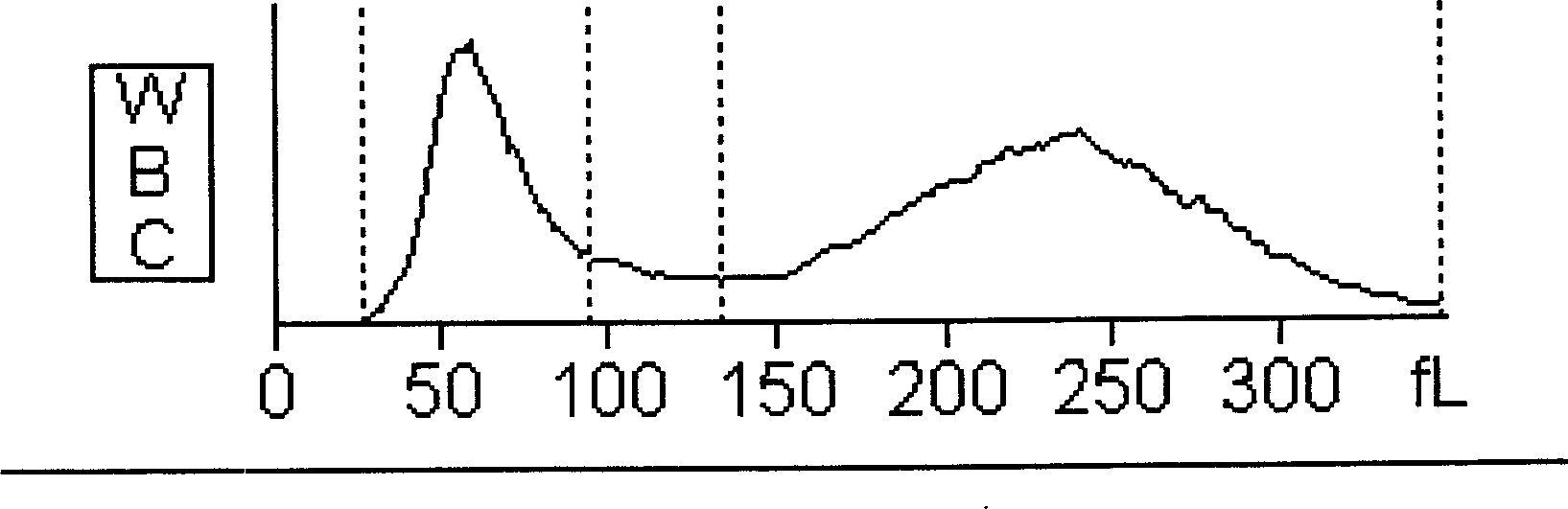
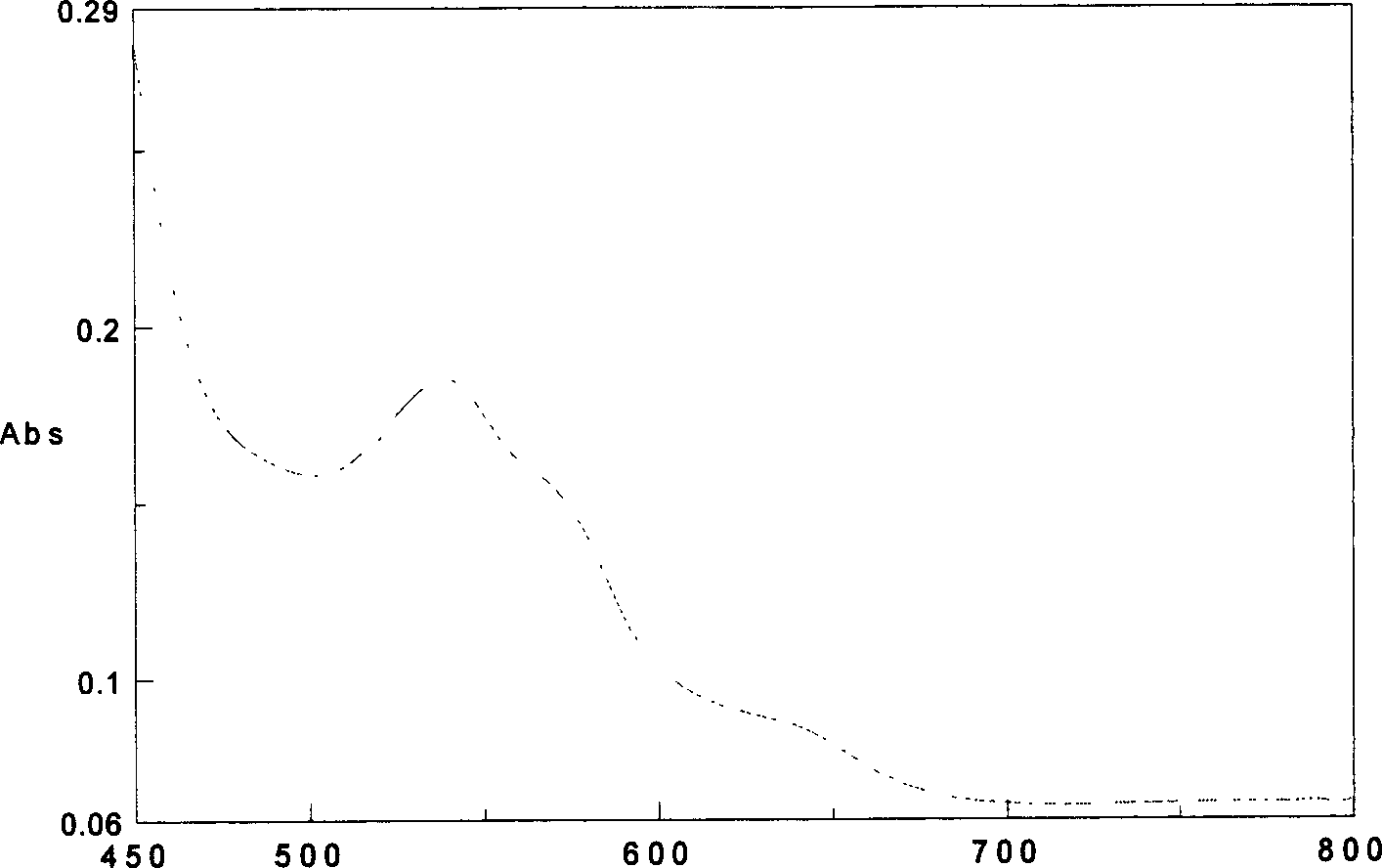
The present invention provides a reliable method to determine lysin of ferrohemoglobin, and meanwhile white cell can be divided into two subgroups for counted separately. How to use the lisin is also included in the present invention. The lysin with no cyanide includes at least one type of surfactant group dissolve red cell sufficiently and to release magnitude of magnitude ferrohemoglobin, at least one type of positive ion surfactant, at least one type of negative ion surfactant and at least one type of non-ion surfactant.
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD +1
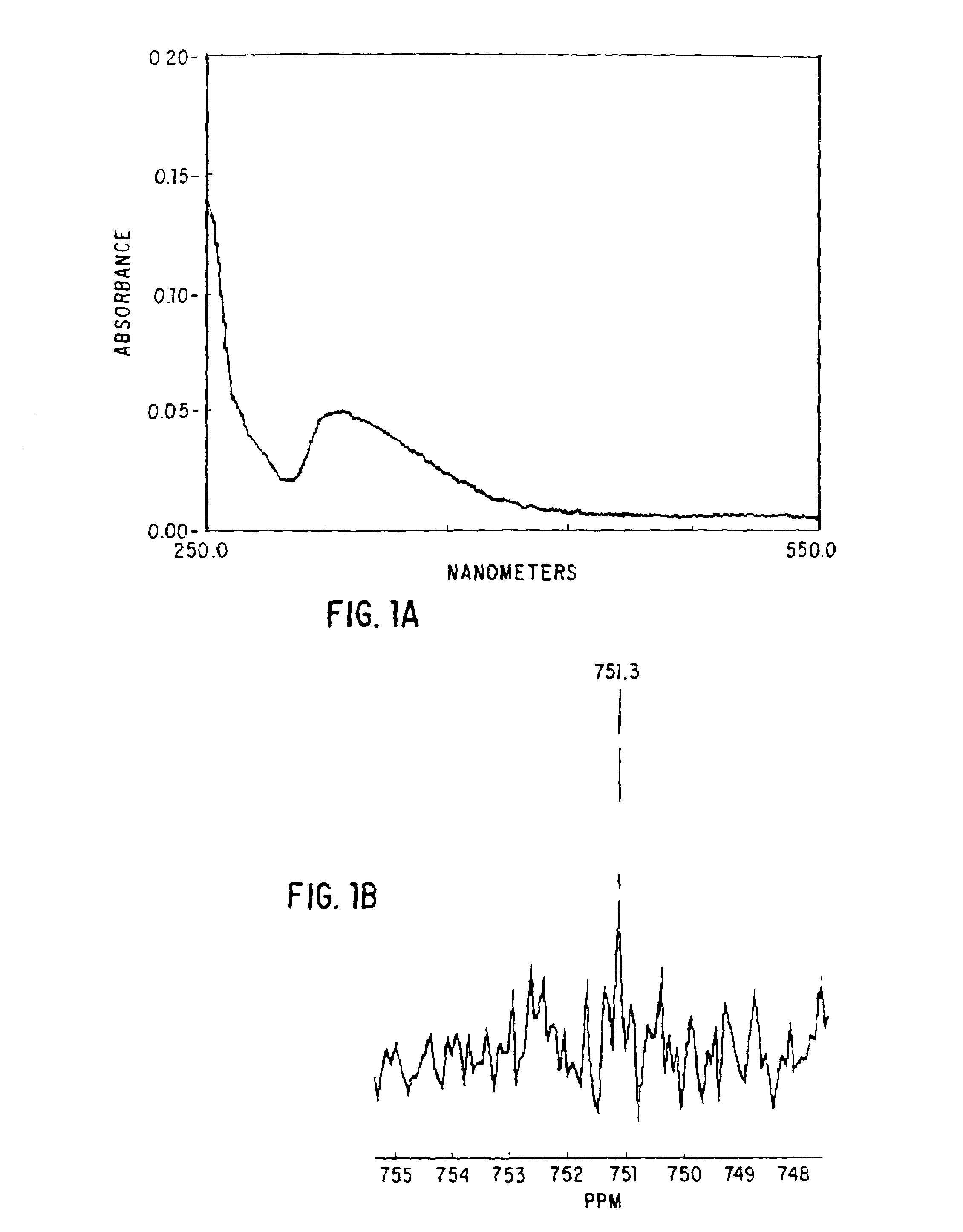
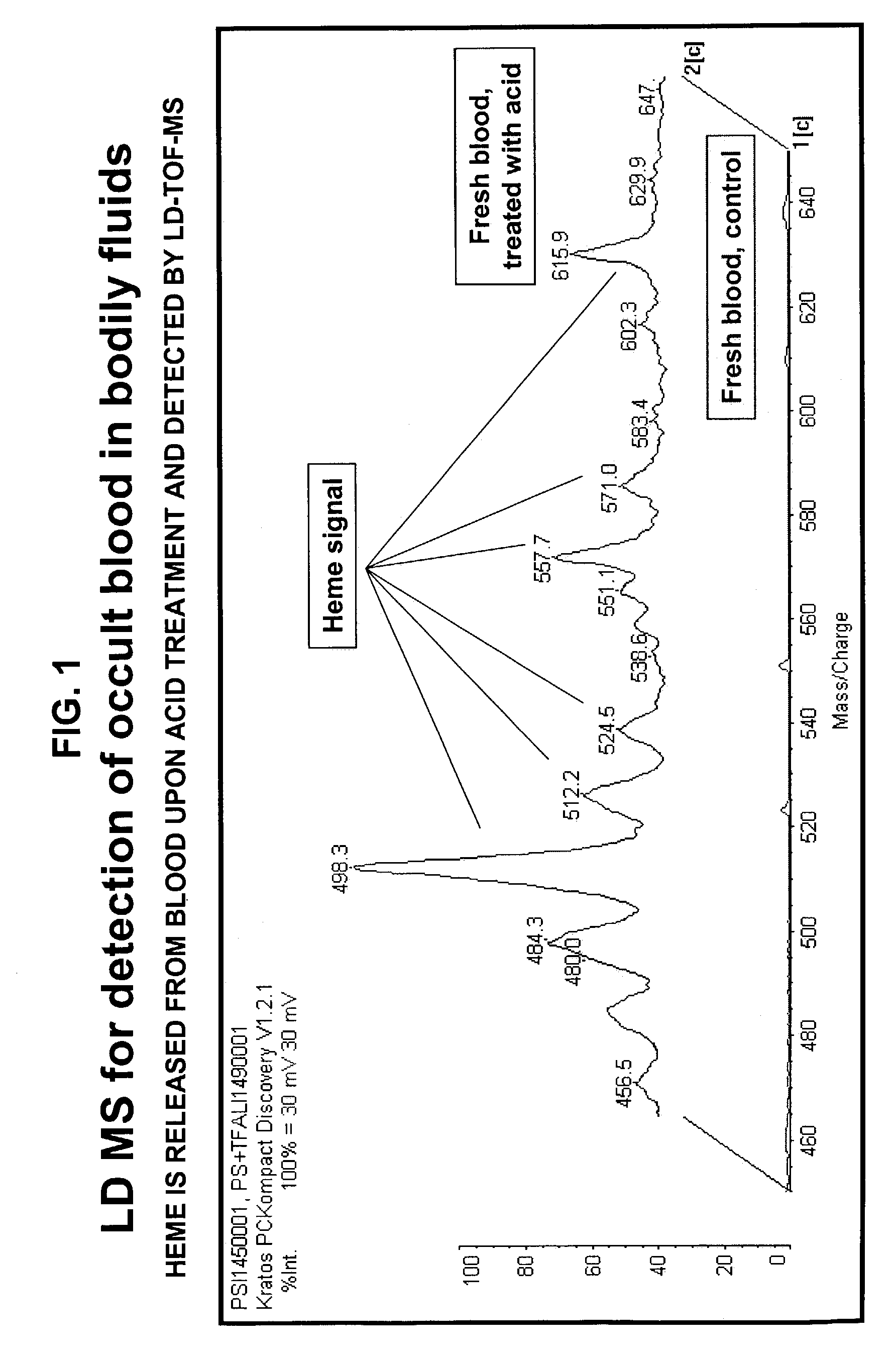
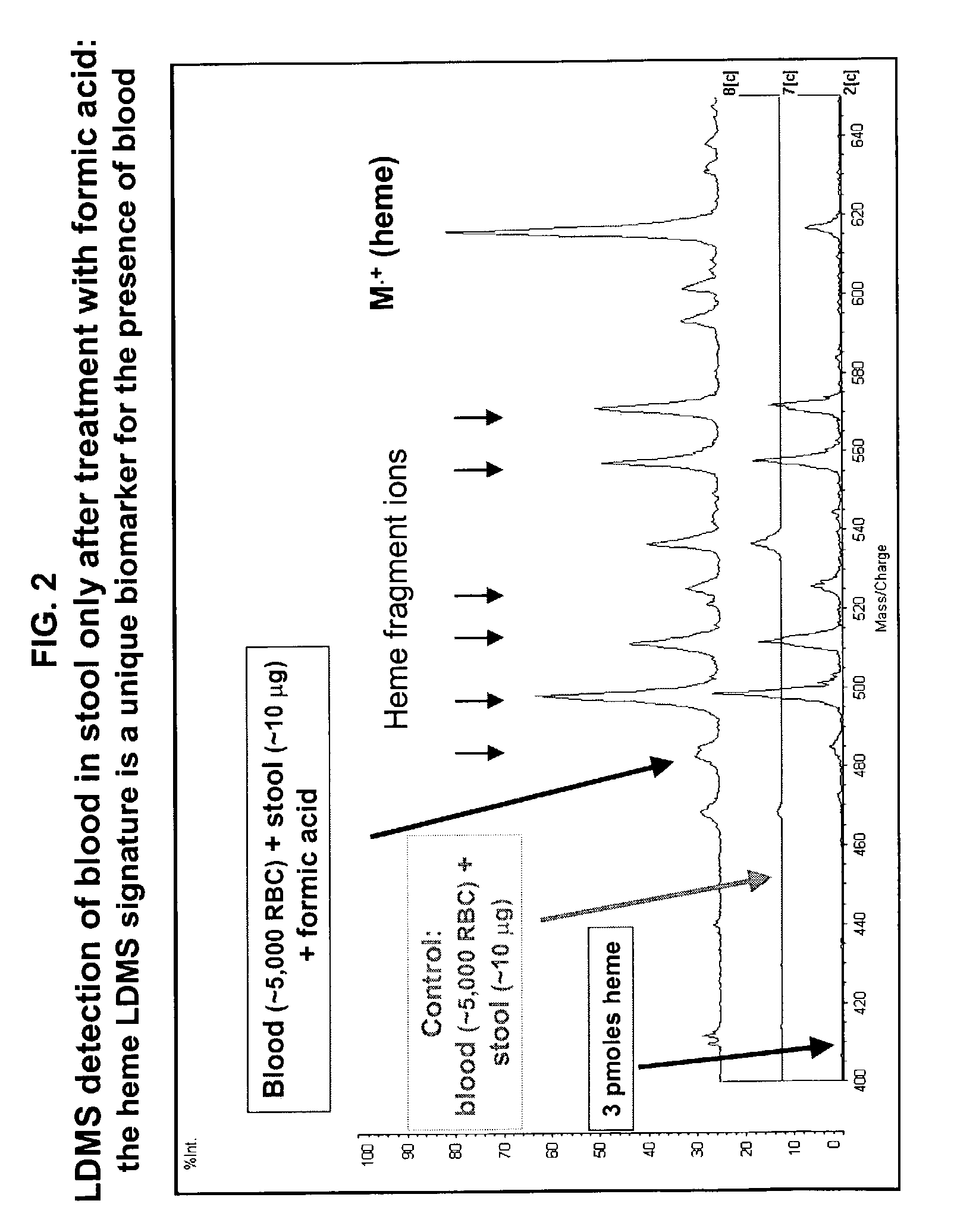
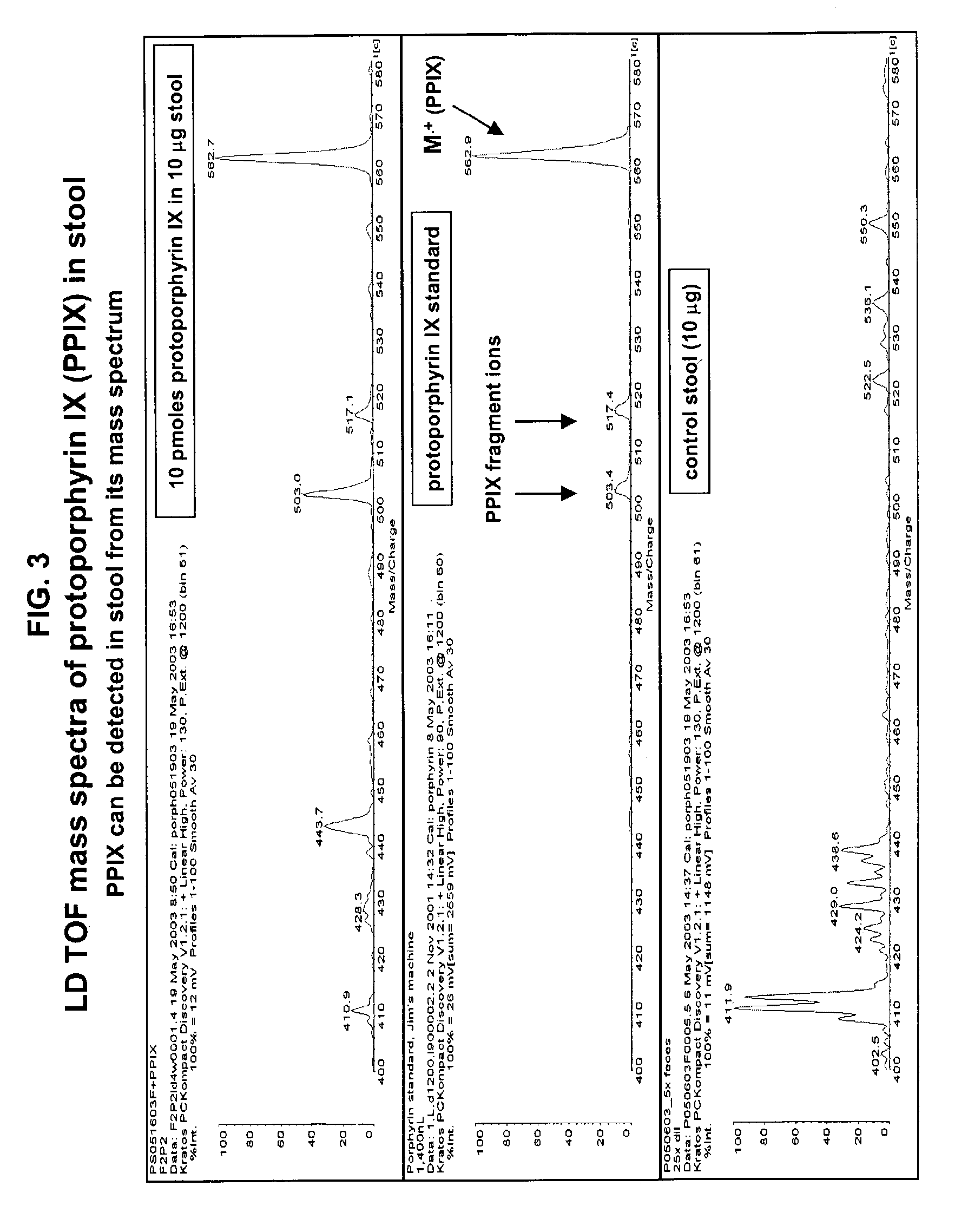
Occult blood detection in biological samples by laser desorption and matrix-assisted laser desorption/ionization mass spectrometry for biomedical applications
Methods are described for detecting and quantifying occult blood in a biological sample using laser desorption mass spectrometry (LD MS). Biological samples that can be analyzed using various embodiments of the present invention include stool (fecal occult blood, FOB), and any bodily fluid including urine, cerebrospinal fluid and other bodily fluids. If the heme or heme metabolite is bound to protein, the sample is treated with acid before analysis to release the porphyrin. Some of the methods use LD MS with a time of flight analyzer (TOF) to detect and measure unbound heme, other hemoglobin metabolites and other molecules that have a porphyrin-based structure, e.g., bilirubin, biliverdin, protoporphyrin IX, and Zinc protoporphyrin in the biological sample. In other methods, matrix-assisted laser desorption / ionization mass spectrometry (MALDI MS) is used to detect and quantify the individual α- and β-polypeptide chains of hemoglobin.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Nitrosylation of protein SH groups and amino acid residues as a therapeutic modality
InactiveUS20060198831A1Inhibiting platelet functionPeptide/protein ingredientsSerum albuminVascular smooth muscle relaxationPlatelet inhibition
Nitrosylation of proteins and amino acid groups enables selective regulation of protein function, and also endows the proteins and amino acids with additional smooth muscle relaxant and platelet inhibitory capabilities. Thus, the invention relates to novel compounds achieved by nitrosylation of protein thiols. Such compounds include: S-nitroso-t-PA, S-nitroso-cathepsin; S-nitroso-lipoprotein; and S-nitroso-immunoglobulin. The invention also relates to therapeutic use of S-nitroso-protein compounds for regulating protein function, cellular metabolism and effecting vasodilation, platelet inhibition, relaxation of non-vascular smooth muscle, and increasing blood oxygen transport by hemoglobin and myoglobin. The compounds are also used to deliver nitric oxide in its most bioactive form in order to achieve the effects described above, or for in vitro nitrosylation of molecules present in the body. The invention also relates to the nitrosylation of oxygen, carbon and nitrogen moieties present on proteins and amino acids, and the use thereof to achieve the above physiological effects.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
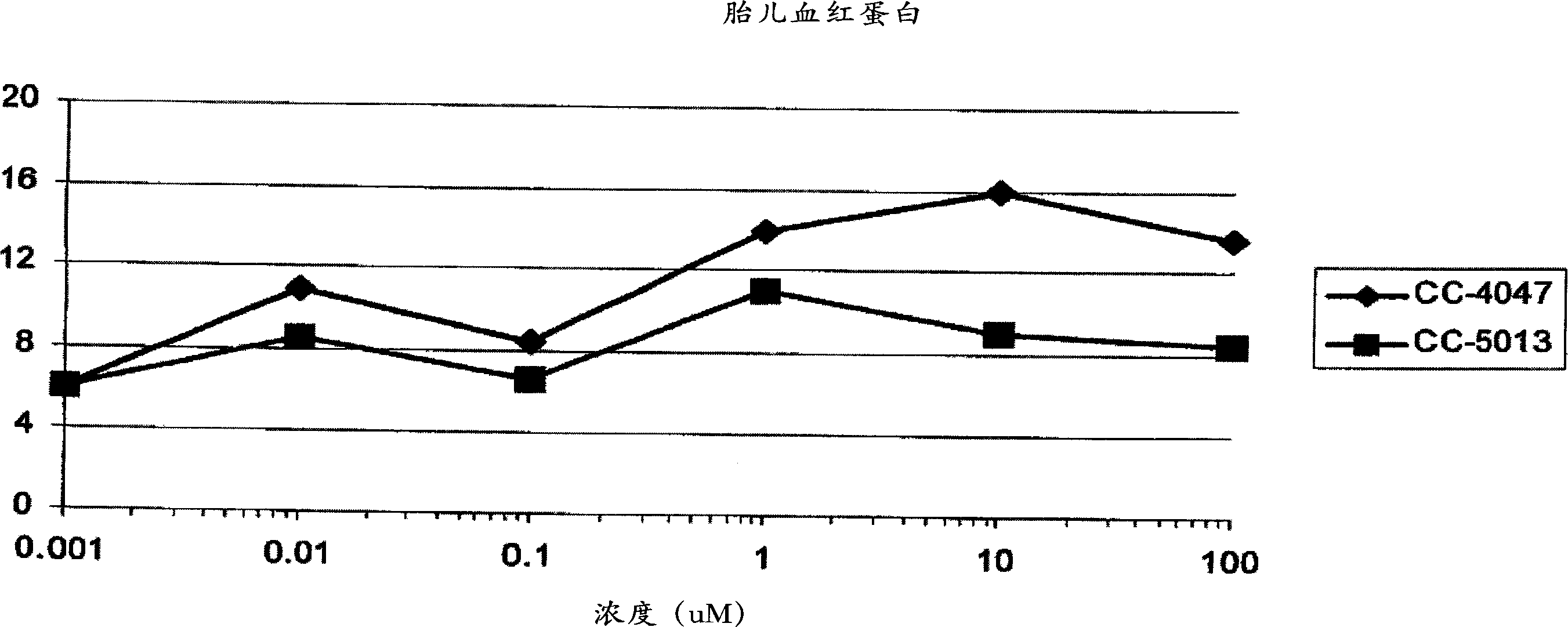
Methods and compositions for the treatment and management of hemoglobinopathy and anemia
InactiveCN1913896AOrganic active ingredientsPeptide/protein ingredientsBeta thalassemiaSickle cell anemia
Owner:CELGENE CORP
Cross-linked hemoglobin preparation method and organ perfusate comprising hemoglobin
InactiveCN110563836AIncrease join speedReduce complexityAntibody mimetics/scaffoldsHaemoglobins/myoglobinsLiquid ChangeCross-link
The invention discloses a cross-linked hemoglobin preparation method, wherein the preparation method comprises the following steps: a, diluting deoxidized hemoglobin, and adding the diluted deoxidizedhemoglobin into an anaerobic reactor; b, arranging an atomizer at the top of the anaerobic reactor, adding a cross-linking agent into the reactor through the atomizer, and carrying out a cross-linking reaction; and c, adding a reducing agent to terminate the cross-linking reaction, and removing the unreacted cross-linking agent and the terminating reducing agent in an ultrafiltration liquid changing manner to obtain the cross-linked hemoglobin. The cross-linked hemoglobin prepared by the preparation method contains low contents of macromolecules (greater than 500 kD) and micromolecules (lessthan 64 kD); by adding the cross-linked hemoglobin into organ perfusate, the phenomena of organ acidosis and lysosomal enzyme activation adverse consequences caused by lack of an oxygen carrying agentin conventional perfusate and cell damage caused by excessive free radicals generated during reperfusion can be solved, and the perfusate is quite simple and convenient to prepare, store, transport and use.
Owner:REDPHARM BEIJING BIOPHARMACEUTICAL INST CO LTD
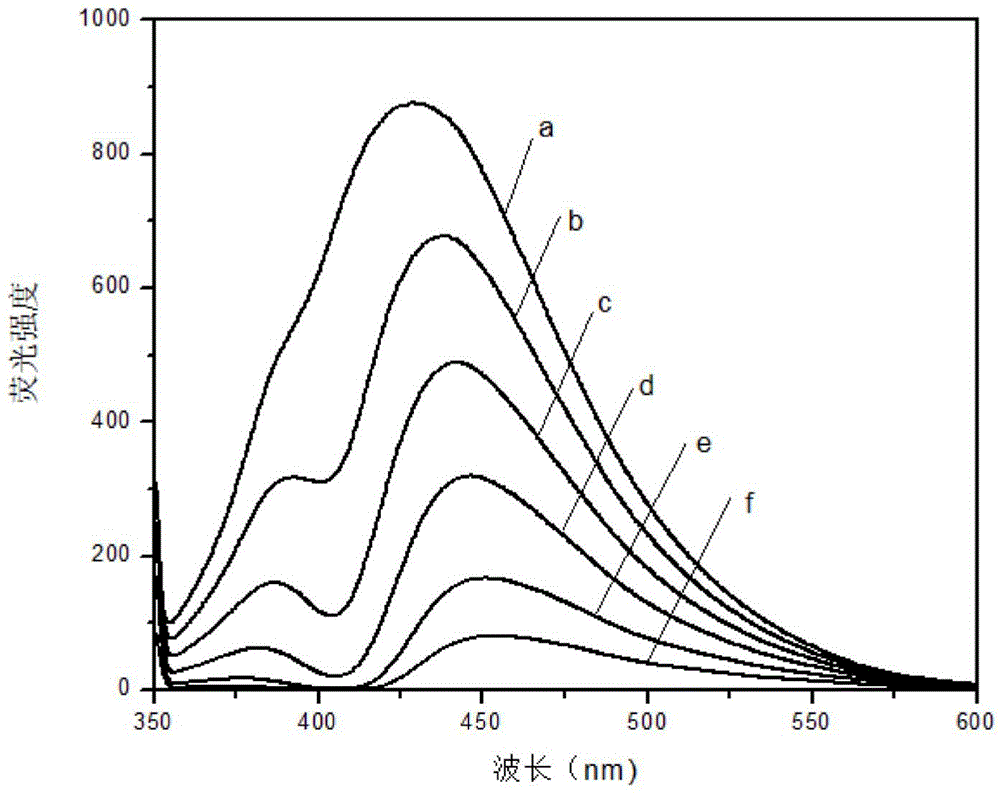
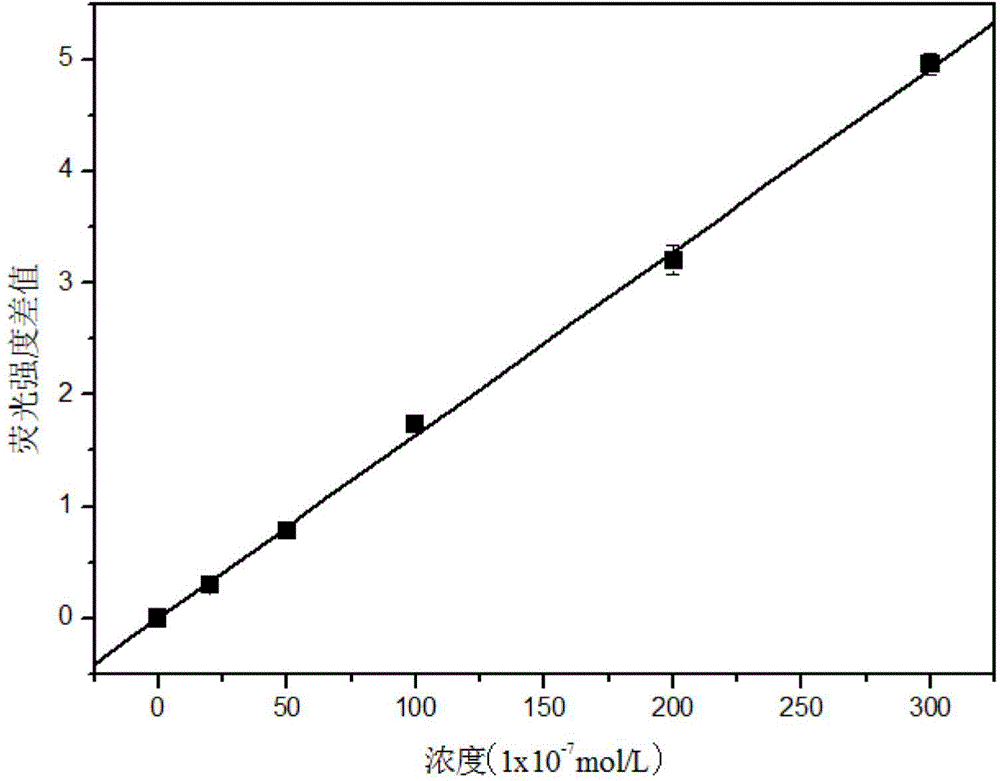
Method for detecting concentration of ferrohemoglobin by using fluorescent carbon dot probe
InactiveCN104964956ALow cytotoxicityGood biocompatibilityFluorescence/phosphorescenceFluorescence spectrometryHemeprotein
The invention discloses a method for detecting the concentration of ferrohemoglobin by using a fluorescent carbon dot probe. The method comprises the following steps: 1, preparing different concentrations of fluorescent carbon dot-containing ferrohemoglobin standard solutions, detecting the fluorescence intensity of the standard solutions to obtain a standard solution fluorescence spectrogram, and establishing a linear relation between a difference between the fluorescence intensity of the standard solution with the ferrohemoglobin concentration being 0 and the fluorescence intensity of every standard solution and the ferrohemoglobin concentrations; and 2, preparing a fluorescent carbon dot-containing ferrohemoglobin sample solution, detecting the fluorescence intensity of the sample solution, and determining the concentration of ferrohemoglobin in the sample solution through the linear relation. The ferrohemoglobin concentration is detected by using the characteristics of a ferrohemoglobin quenching fluorescence carbon dot with the fluorescence carbon dot as a probe, so the method has the advantages of simple and convenient detection process, high sensitivity and low detection limit, and can realize online, in-situ, rapid and sensitive detection of the concentration of ferrohemoglobin in a practical sample.
Owner:GUANGXI TEACHERS EDUCATION UNIV
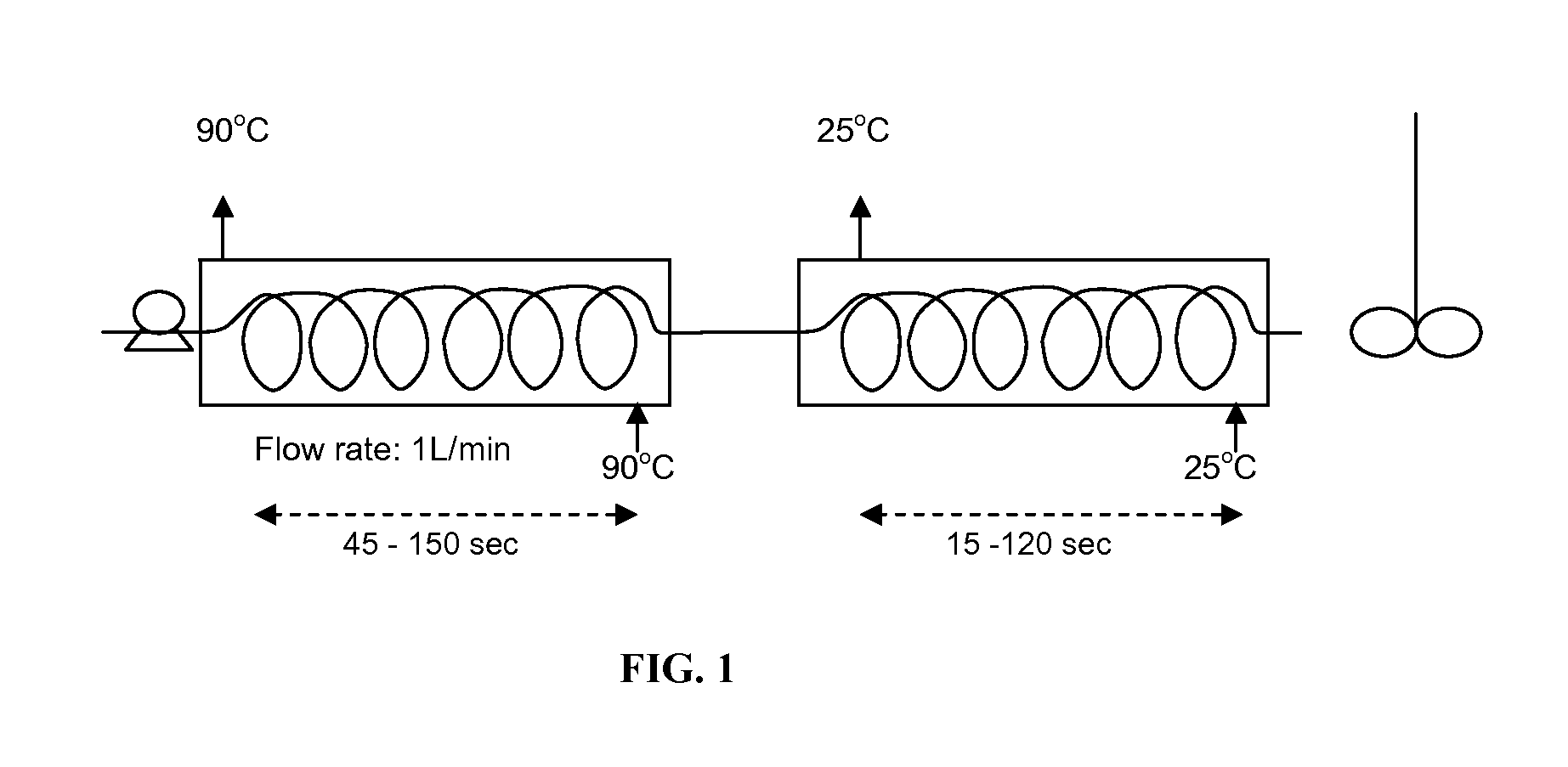
Method for removing unmodified hemoglobin from cross-linked hemoglobin solutions including polymeric hemoglobin with a high temperature short time heat treatment apparatus
ActiveUS8084581B1Improved intravascular retention timeReduction and elimination of significant renal eliminationPeptide/protein ingredientsHaemoglobins/myoglobinsPhysical chemistrySerum albumin
A method heat treatment of cross-linked hemoglobin solutions including polymeric hemoglobin is disclosed. The method involves contacting the hemoglobin solution with a high temperature short time heat treatment apparatus. The high temperature short time process thermally denatures unmodified tetrameric hemoglobin (hemoglobin dimer form), protein impurities (e.g. immunoglobin-G, serum albumin), bacteria, and viruses so that renal injury, vascular detrimental effects and other toxicity reactions can be avoided.
Owner:BILLION KING INT
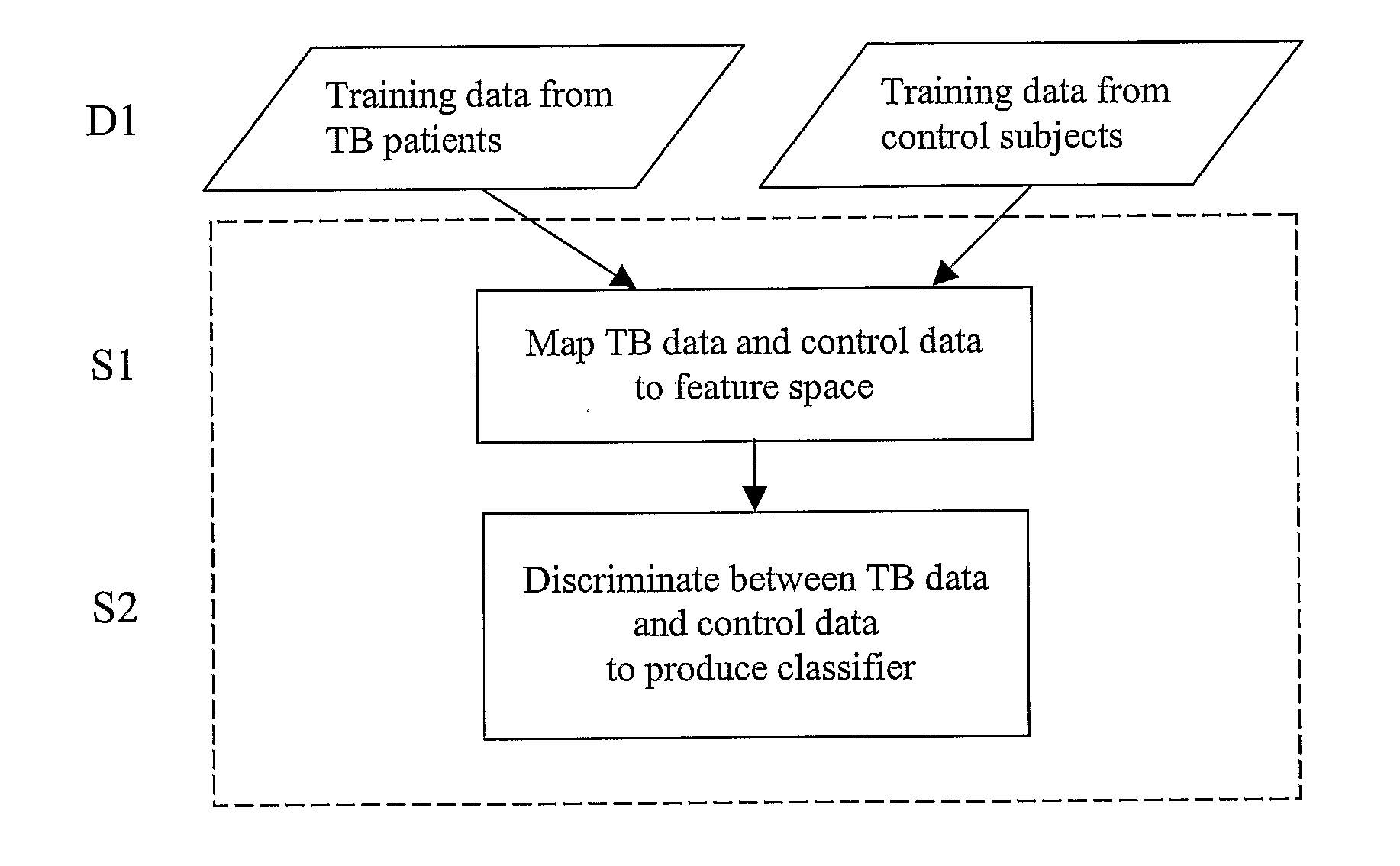
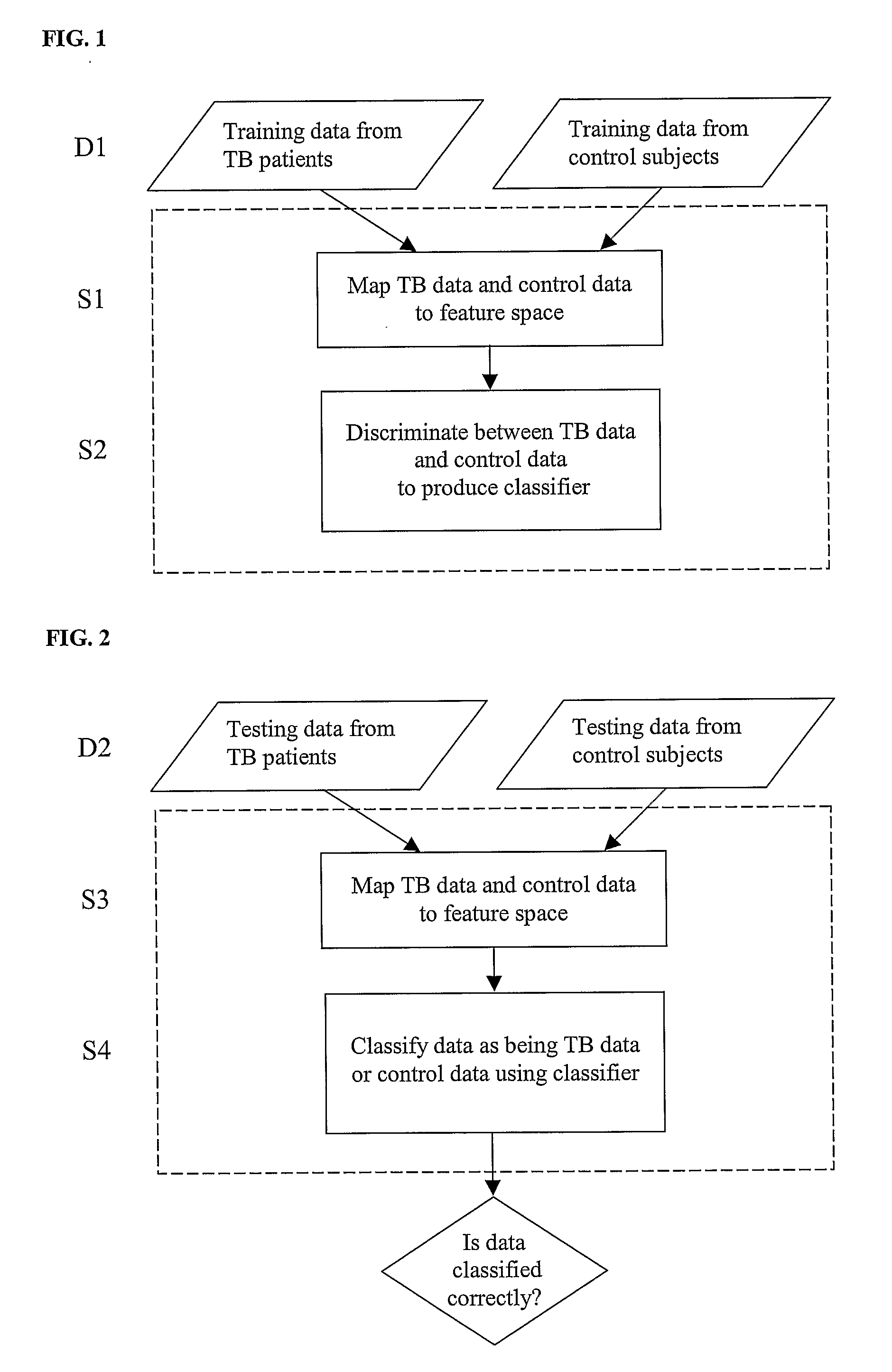
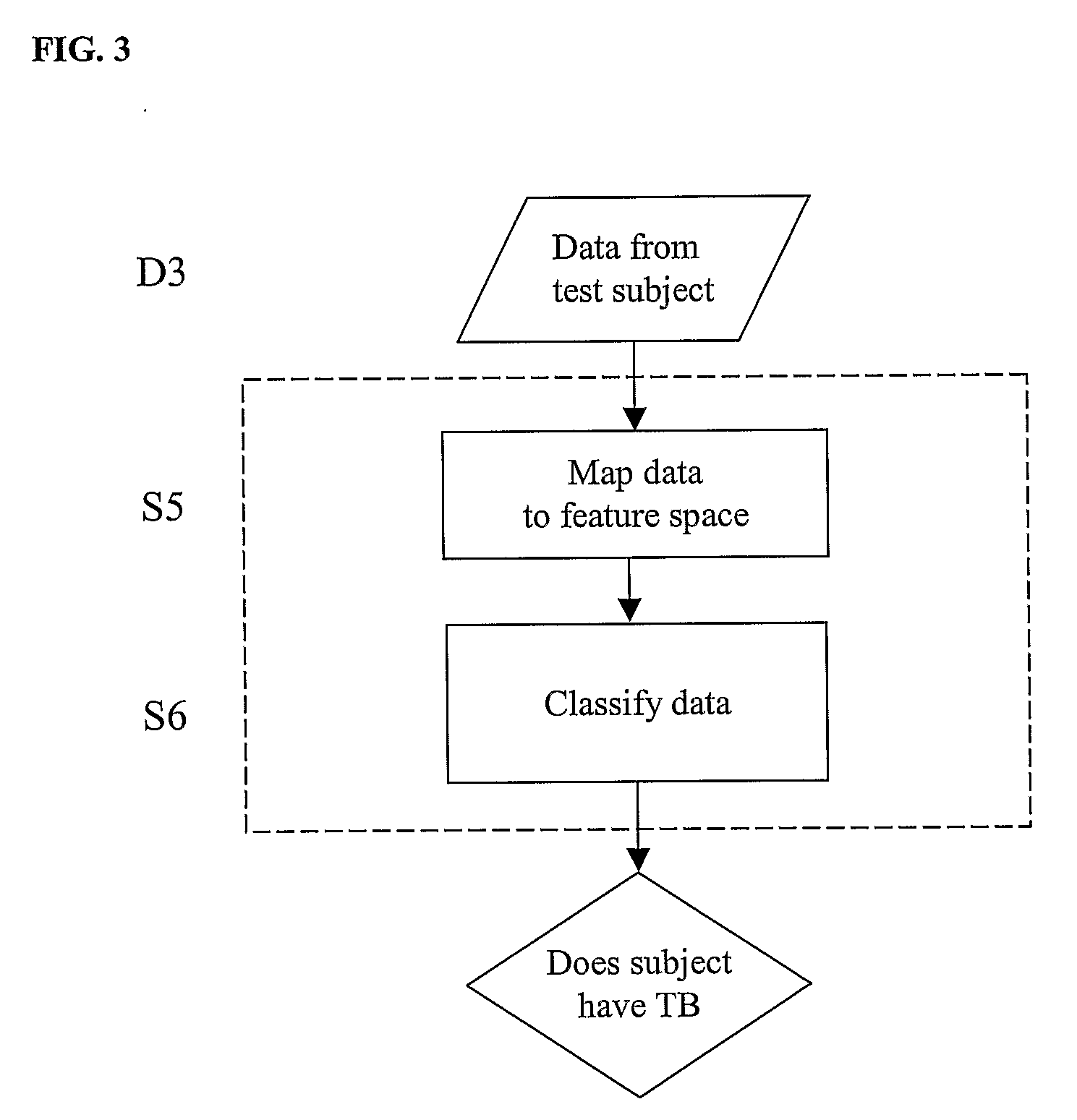
Diagnosis of Tuberculosis
InactiveUS20090104602A1Microbiological testing/measurementMaterial analysisSerum protein albuminApolipoproteins E
The invention provides a method of diagnosing tuberculosis (TB) in a test subject, said method comprising: (i) providing expression data of two or more markers in a subject, wherein at least two of said markers are selected from transthyretin, neopterin, C-reactive protein (CRP), serum amyloid A (SAA), serum albumin, apoliopoprotein-A1 (Apo-A1), apolipoprotein-A2 (Apo-A2), hemoglobin beta, haptoglobin protein, DEP domain protein, leucine-rich alpha-2-glycoprotein (A2GL) and hypothetical protein DFKZp667I032; and (ii) comparing said expression data to expression data of said marker from a group of control subjects, wherein said control subjects comprise patients suffering from inflammatory conditions other than TB, thereby determining whether or not said test subject has TB.
Owner:FERNANDEZ REYES DELMIRO +3
Process for preparing low molecular peptide and amino acid by biological enzymolysis of pig blood haematoglobin
InactiveCN1858228AGreat tasteImprove solubilityHaemoglobins/myoglobinsPeptide preparation methodsSolubilityHemeprotein
The present invention relates to the process of biologically enzymolyzing pig blood haematoglobin to prepare small molecular weight peptide and amino acid. The process includes adding water to pig blood haematoglobin to regulate protein content to 11.4-16.5 wt%, regulating pH value to 7.2-7.7, maintaining temperature at 50-55 deg.c adding mixture proteinase of pancereatin, Protamex and Flavourzyme or papain in the amount of 330-720 U / g to react for 12-17 hr to reach hydrolysis degree of 24-30, and centrifugally separating to eliminate insoluble part. The present invention adopts cell breaking technology, and has optimized combination of composite proteinase and optimized reaction condition to reach protein recovering rate up to 90-95%. The product has high solubility, high foamability, outstanding wet keeping performance and no bad smell and may be used widely in food production.
Owner:SOUTH CHINA UNIV OF TECH
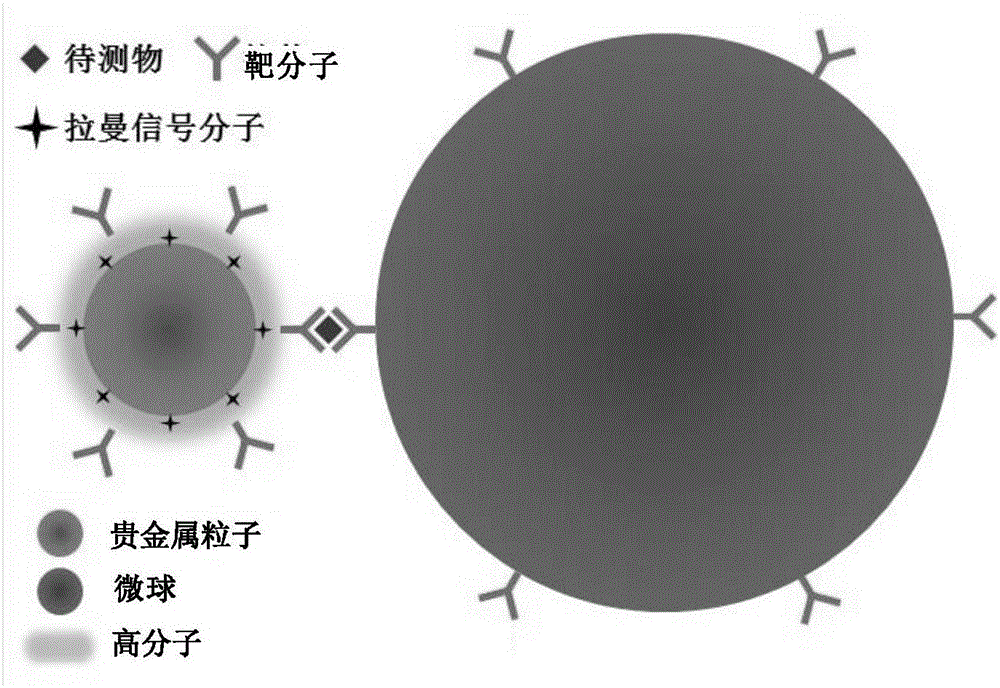
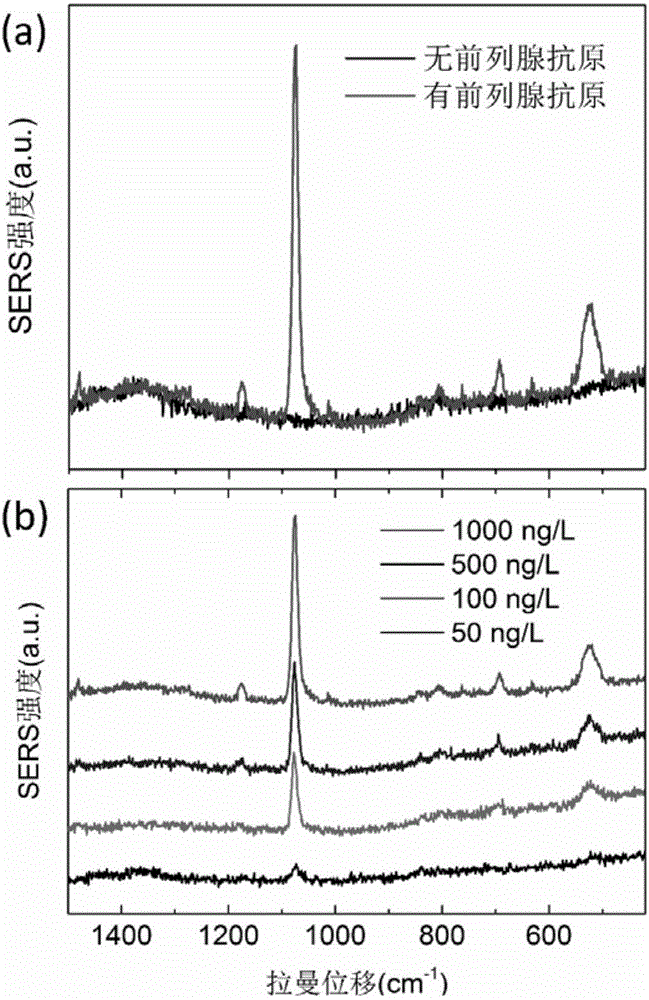
Surface enhanced Raman scattering technology-based composite material and preparation method thereof
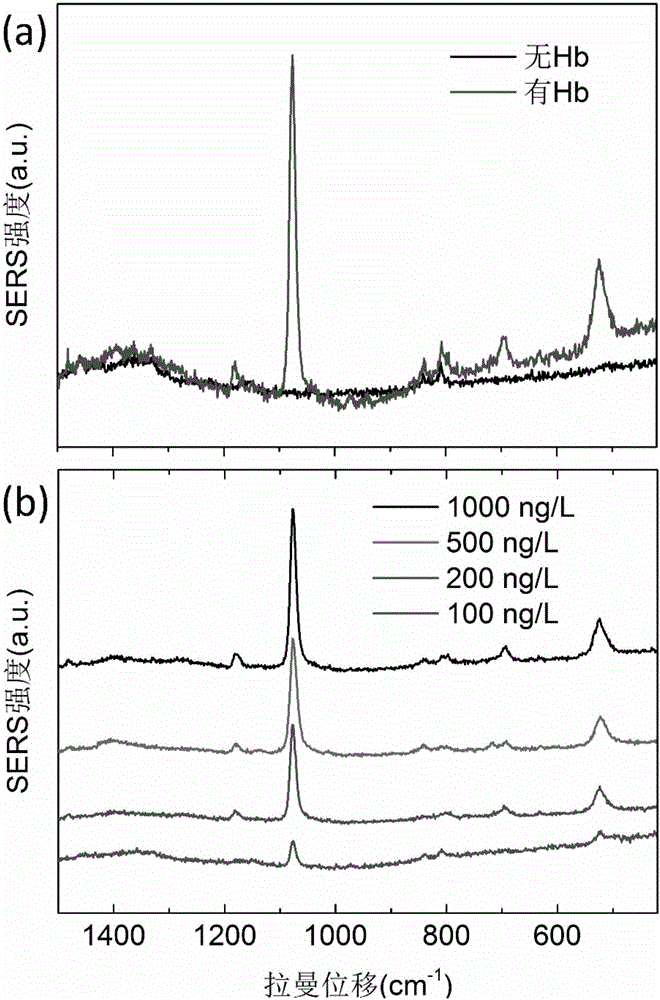
The invention relates to a surface enhanced Raman scattering technology-based composite material and a preparation method thereof. The composite material comprises microspheres modified with first target molecules on the surfaces and a second component modified with Raman signal molecules and second target molecule-modified polymers on the surface. In a solution containing a substance to be detected, the substance to be detected, the microspheres and the second component are bonded to the second target molecules through the first target molecules so that the composite material which can be easily precipitated through centrifugation is obtained. The invention also discloses the preparation method and a use of the composite material. The composite material can be used for detecting a human prostate-specific antigen (PSA), a human ferrohemoglobin (Hb), a procalcitonin (PCT) or disease markers (such as tumor markers, cardiovascular disease markers, senile dementia markers, mycoplasma and chlamydia), can effectively reduce false positive and false negative effects and has advantages of high sensitivity, simpleness, fastness and low cost.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
A cyanide-free lytic reagent compositiona nd method for hemoglobin and cell analysis
The present invention relates to a cyanide-free lytic reagent composition and method for measuring the total hemoglobin concentration in a blood sample, for counting the number of leukocytes and for differential counting of three leukocyte subpopulations including lymphocytes, monocytes, and granulocytes. The cyanide-free lytic reagent composition comprises a hemolytic surfactant selected from the group consisting of quaternary ammonium salts, pyridinium salts, organic phosphate esters, and alkyl sulfonates to lyse erythrocytes and release hemoglobin, and an organic ligand selected from the group consisting of triazole and its derivatives, tetrazole and its derivatives, alkaline metal salts of oxonic acid, melamine, aniline-2-sulfonic acid, quinaldic acid, 2-amino-1,3,4-thiadiazole, triazine and its derivatives, urazole, DL-pipecolinic acid, isonicotinamide, anthranilonitrile, 6-aza-2-thiothymine, adenine, 3-(2-thienyl)acrylic acid, benzoic acid and alkali metal and ammonium salts of benzoic acid, and pyrazine and its derivatives to form a stable chromogen with hemoglobin. Alternatively, the organic ligands can be added to a suitable blood diluent for hemoglobin and leukocyte analysis.
Owner:COULTER INTERNATIONAL CORPORATION
Medical use of the radical scavenger and antioxidant alpha-1-microglobulin
Medical use of alpha-1-microglobulin (A1M) in the treatment or prophylaxis of diseases wherein oxidative stress is a responsible factor in the progress of the disease. Notably, the present invention relates to the medical use of alpha-1-microglobulin in the treatment or prophylaxis of diseases or conditions associated with the presences of free radicals and / or free haemoglobin in the subject.
Owner:GUARD THERAPEUTICS INT AB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com