Chemokine receptor binding heterocyclic compounds with enhanced efficacy
A compound and effective dose technology, applied in the directions of active ingredients of heterocyclic compounds, medical preparations containing active ingredients, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
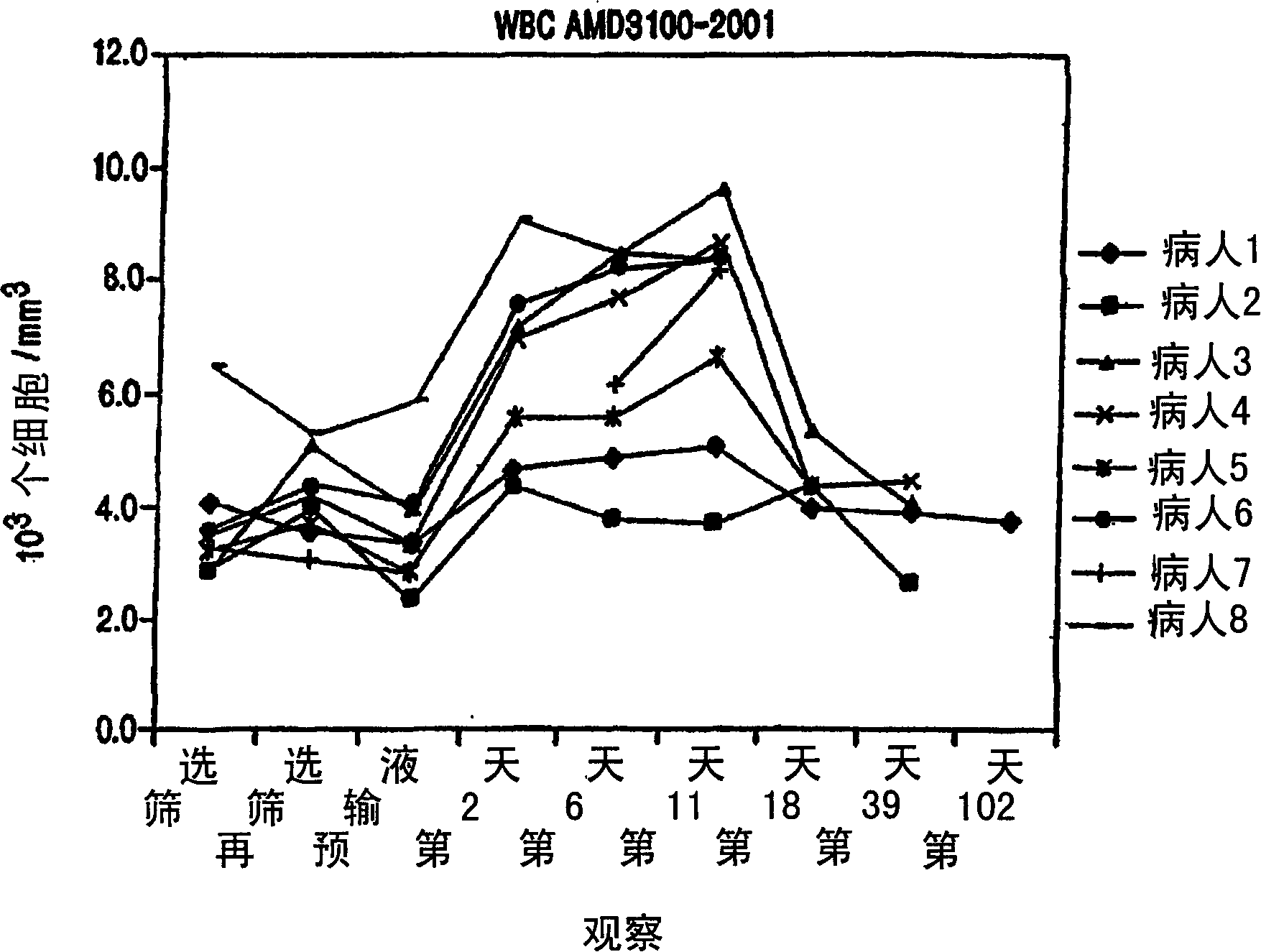
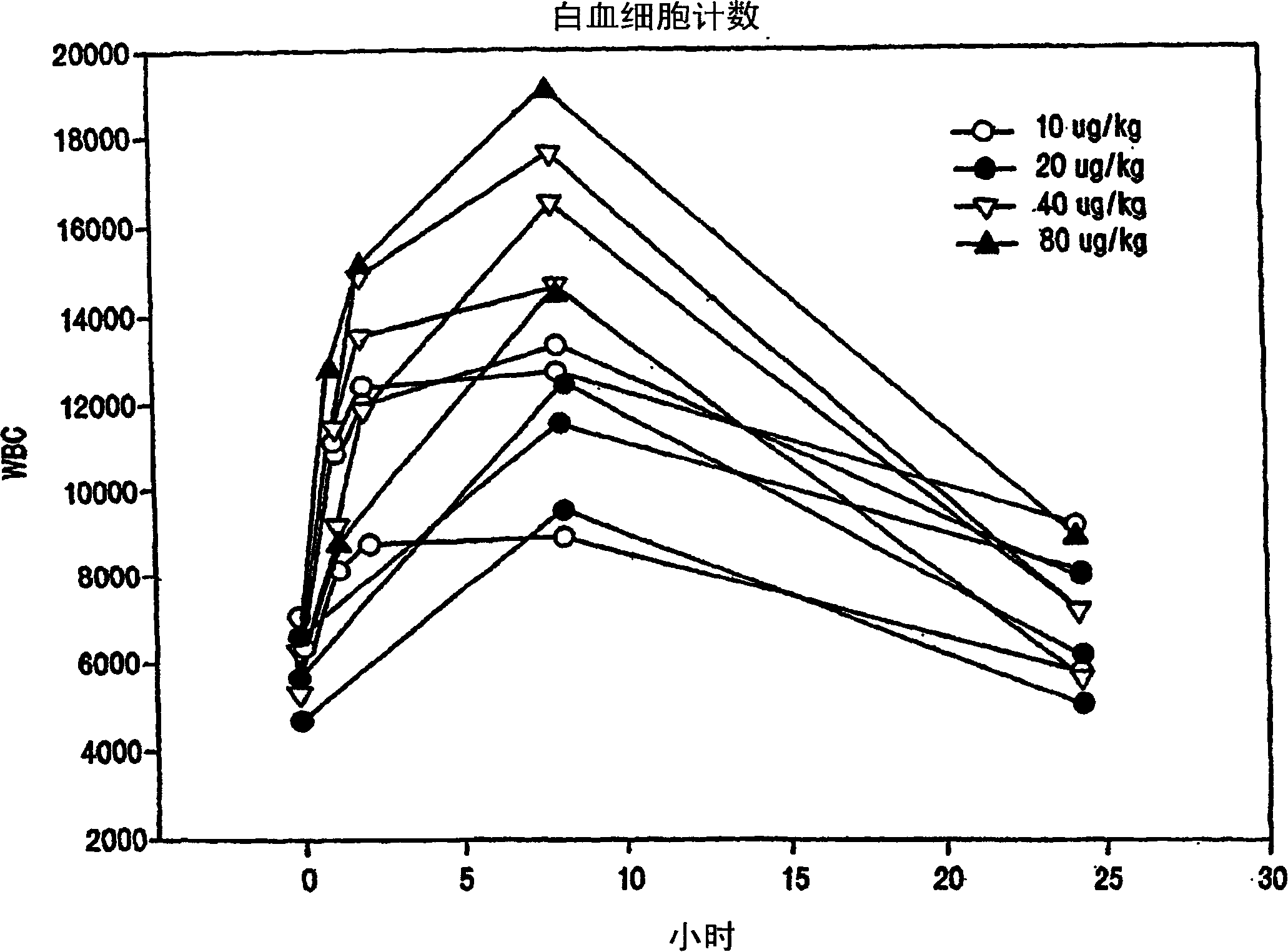
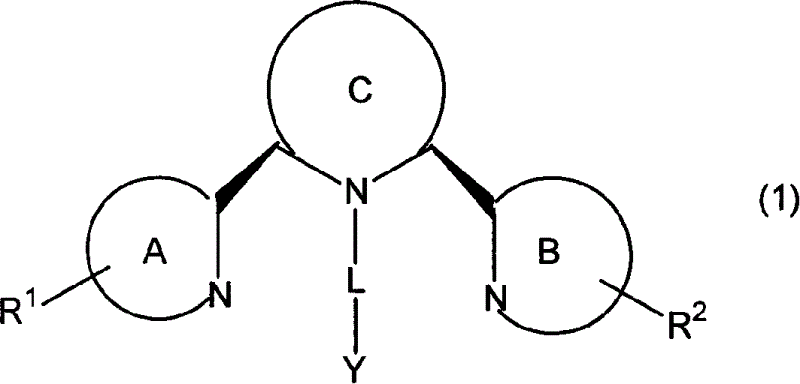
Image
Examples
Embodiment 1
[0210]
[0211] Compound 1:
[0212] 4-(3,3"-dimethyl-3',4',5',6'-tetrahydro-2'H-cis-[2,2';6',2"]terpyridine-1' -yl)-butylamine
[0213] To a cold (-78°C) solution of N,N,N',N'-tetramethylethylenediamine (TMEDA) (4.06 mL, 26.9 mmol) in dry THF (50 mL) under Ar atmosphere, n-butyl Lithium (2.5M in hexane, 10.7 mL, 26.9 mmol). 2-Bromo-3-methylpyridine (3.0 mL, 26.9 mmol) was added dropwise, and the temperature was raised to -55° C. for 30 min. The reaction mixture turned red. It was then cooled to -78°C and dimethyl glutarate (1.65 mL, 11.2 mmol) was added. The reaction mixture was stirred at -78 °C for 1 h. Water (200mL) was added, and CH 2 Cl 2 The mixture was extracted (3 times, 200 mL). The combined organic extracts were washed with saturated brine (200 mL), and passed through MgSO 4 Dry, filter and concentrate. The crude product was purified by flash chromatography on silica gel (1:1 EtOAc:hexanes) to afford 1.5 g (48%) of 1,5-bis-(3-methyl-pyridin-2-yl) as a w...
Embodiment 2
[0221]
[0222] Compound 2:
[0223] [3-aminomethyl-4-meso-(3,3"-dimethyl-3',4',5',6'-tetrahydro-2'H-cis-[2,2'; 6',2"]terpyridine-1'-ylmethyl)-phenyl]-methanol
[0224] At 0°C, to a solution of 3-picolinecarbaldehyde (43.27g, 357mmol) in MeOH (179mL) was added NH 4OAc (151.14 g, 197 mmol). 1,3-Acetonedicarboxylic acid (26.10 g, 178.6 mmol) was then slowly added to the reaction over 15 min. After a large number of bubbles were eliminated, the solution was warmed to room temperature for 1 h while stirring. The solvent was removed under reduced pressure and CH was added 2 Cl 2 (500mL). The solution is saturated with Na 2 CO 3 Aqueous solution (350 mL) was washed and separated. Aqueous phase with CH 2 Cl 2 (2×400mL) extraction, the combined organic components were dissolved in Na 2 SO 4 Dried and concentrated under reduced pressure, purified by flash chromatography on a silica gel plug (2:0.5:97.5 MeOH / NH 4 OH / CH 2 Cl 2 ), resulting in yellow meso-3,3"-dimethyl-...
Embodiment 3
[0234]
[0235] Compound 3: (1-[4-((2′S,6′R)-3,3″-dimethyl-3′,4′,5′,6′-tetrahydro-2′H-[2 ,2'; 6',2"]terpyridin-1'-yl)butyl]-3-(1H-imidazol-2-yl)-urea
[0236] CH 2 Cl 2 (10 mL) the solution was stirred for 5 h, then the solvent was removed. The residue was dissolved in DMF (6 mL), and 4-((2'S,6'R)-3,3"-dimethyl-3',4',5',6'-tetrahydro-2' was added H-[2,2';6',2"]terpyridin-1'-yl)-butylamine (0.130 g, 0.384 mmol) and DIPEA (0.293 g, 2.27 mmol). The mixture was heated at 75 °C for 16 h, then cooled to room temperature. Add saturated NaHCO 3 aqueous solution (20 mL), and the mixture was distilled with CH 2 Cl 2 (3 x 30 mL) extraction. The combined extracts were treated with Na 2 SO 4 dry. After filtration, the solvent was evaporated under vacuum, and the residue was purified by flash chromatography on a silica gel column (100:5:2 CH 2 Cl 2 / CH 3 OH / NH 4 OH), with CH2Cl 2 / hexane precipitation and evaporation in vacuo to give a pale yellow solid (0.115 g, 67%). 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com