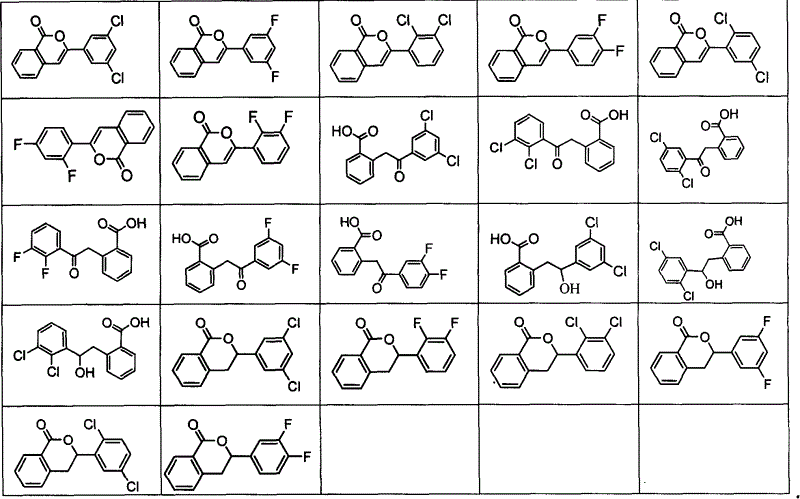
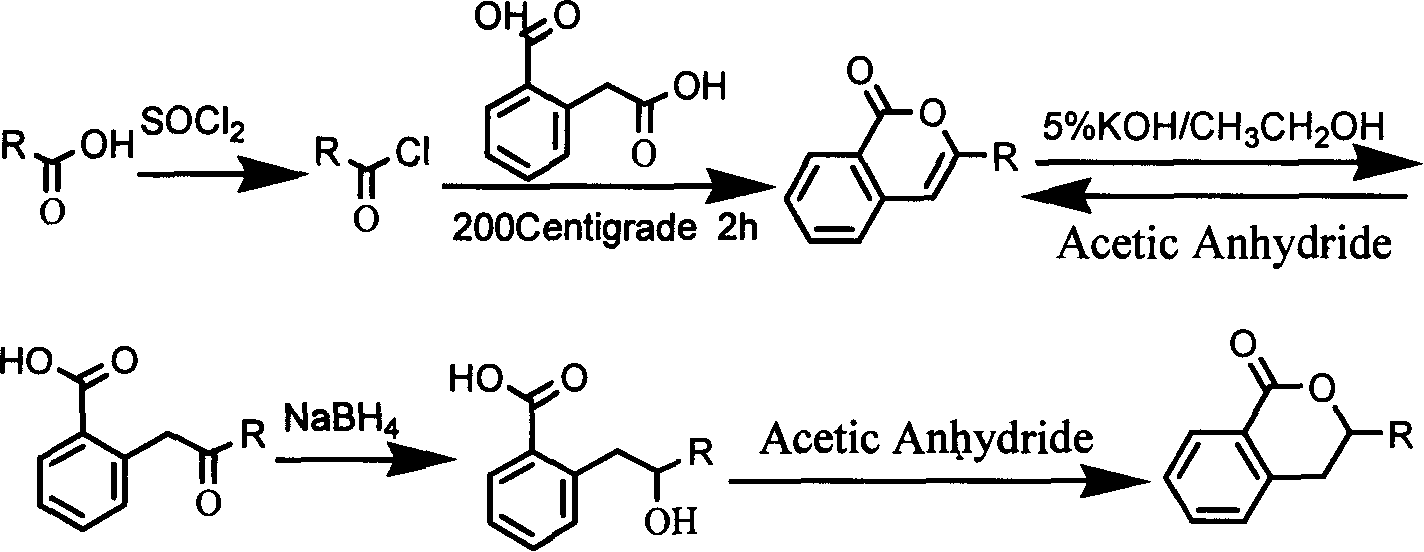
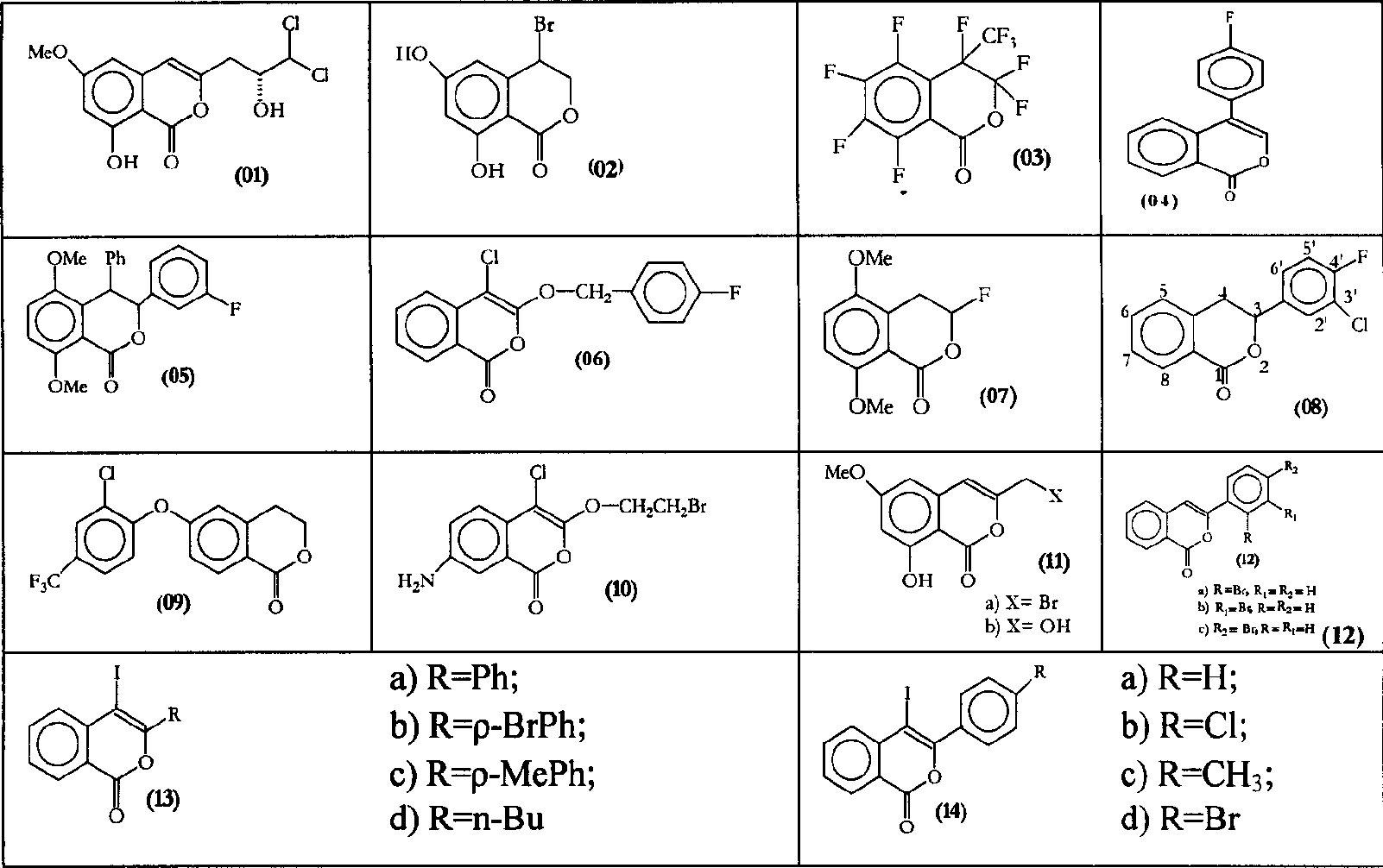
Preparation method of non natural isocumarin and its 3,4-dihydro derivative and use thereof
A technology of isocoumarin and its derivatives, which can be used in the fields of chemicals for biological control, biocides, and resistance to vector-borne diseases, etc., and can solve the problems of relatively few biological activity reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 3, the preparation of 5-dichlorobenzoyl chloride (31'):
[0046] Add 3,5-dichlorobenzoic acid (31) (10.0g, 53.0mmol) and thionyl chloride (4.5ml, 38.0mmol) in a 500mL three-necked flask, add dropwise DMF to the mixture, and reflux for 30 minutes. When gas was generated, it was proved that the reaction was over, and the unreacted thionyl chloride was removed under reduced pressure to obtain dihalobenzoyl chloride (11 g, 50.0 mmol) (31'). The product was stored in a desiccator without purification and was used directly for the next reaction.
Embodiment 2
[0048] The preparation of 2,3-dichlorobenzoyl chloride (32'):
[0049] Add 2,3-dichlorobenzoic acid (32) (10.0g, 53.0mmol) and thionyl chloride (4.5ml, 38.0mmol) in a 500mL three-necked flask, add dropwise DMF to the mixture, and reflux for 30 minutes. When gas was generated, it was proved that the reaction was over, and the unreacted thionyl chloride was removed under reduced pressure to obtain dihalobenzoyl chloride (11 g, 50.0 mmol) (32'). The product was stored in a desiccator without purification and was used directly for the next reaction.
Embodiment 3
[0051] The preparation of 2,5-dichlorobenzoyl chloride (33'):
[0052] Add 2,5-dichlorobenzoic acid (33) (10.0g, 53.0mmol) and thionyl chloride (4.5ml, 38.0mmol) in a 500mL three-necked flask, add dropwise DMF to the mixture, and reflux for 30 minutes. When gas was generated, it was proved that the reaction was over, and the unreacted thionyl chloride was removed under reduced pressure to obtain dihalobenzoyl chloride (11 g, 50.0 mmol) (33'). The product was stored in a desiccator without purification and was used directly for the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com