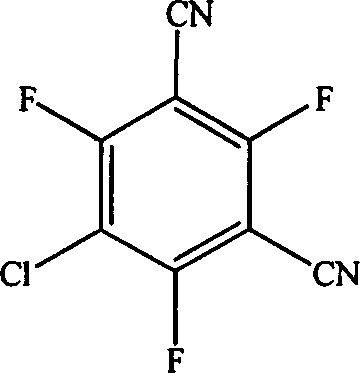
Gas chromatography analysis method for 5-chloro-2,4,6-trifluoro-1,3-bezenedicarbonitrile
A technology for gas chromatography analysis and chlorothalonil, applied in analytical materials, chemical instruments and methods, material separation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1. Preparation of internal standard solution
[0025] Weigh 50mg (accurate to 1mg) of dimethyl phthalate in a 100mL volumetric flask, dissolve it with acetone and make to volume.
[0026] 2. Preparation of standard solution
[0027] Weigh 50 mg (according to pure calculation, accurate to 1 mg) of chlorothalonil standard sample in a 10 mL volumetric flask, add 5 mL of internal standard solution to fully dissolve, dissolve with acetone and make up to volume.
[0028] 3. Preparation of sample solution
[0029] Weigh a chlorothalonil sample containing 50 mg of active ingredients (according to pure calculation, accurate to 1 mg) into a 10 mL volumetric flask, accurately add 5 mL of internal standard solution to fully dissolve, add an appropriate amount of acetone to dissolve, and then ultrasonically oscillate for 5 min. Dilute to volume with acetone, filter and test.
[0030] 4. Determination
[0031] The sample to be tested in step 3 is filled with a ShimaliteW (AW-DMCS...
Embodiment 2
[0033] 1. Preparation of internal standard solution
[0034] Weigh 200mg (accurate to 1mg) of dimethyl phthalate in a 100mL volumetric flask, dissolve it with acetone and make to volume.
[0035] 2. Preparation of standard solution
[0036] Weigh 100mg (according to pure calculation, accurate to 1mg) trifluorochlorothalonil standard sample into a 25mL volumetric flask, add 10mL internal standard solution to fully dissolve, dissolve with acetone and make up to volume.
[0037] 3. Preparation of sample solution
[0038] Weigh the chlorothalonil sample containing equivalent to 100mg active ingredient (according to pure calculation, accurate to 1mg) into a 25mL volumetric flask, accurately add 10mL internal standard solution to fully dissolve, add appropriate amount of acetone to dissolve, and then ultrasonically oscillate for 5min. Dilute to volume with acetone, filter and test.
[0039] 4. Determination
[0040] The sample to be tested in step 3 is filled with a ShimaliteW (...
Embodiment 3
[0042] 1. Preparation of internal standard solution
[0043] Weigh 2000mg (accurate to 1mg) of dimethyl phthalate in a 100mL volumetric flask, dissolve it with acetone and make to volume.
[0044] 2. Preparation of standard solution
[0045] Weigh 2000mg (according to pure calculation, accurate to 1mg) of chlorothalonil standard sample in a 100mL volumetric flask, add 25mL internal standard solution to fully dissolve, dissolve with acetone and make up to volume.
[0046] 3. Preparation of sample solution
[0047] Weigh a chlorothalonil sample containing 2000 mg of active ingredients (according to pure calculation, accurate to 1 mg) into a 100 mL volumetric flask, accurately add 25 mL of internal standard solution to fully dissolve, add an appropriate amount of acetone to dissolve, and then ultrasonically oscillate for 5 min. Dilute to volume with acetone, filter and test.
[0048] 4. Determination
[0049] The sample to be tested in step 3 is filled with a ShimaliteW (AW-D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com