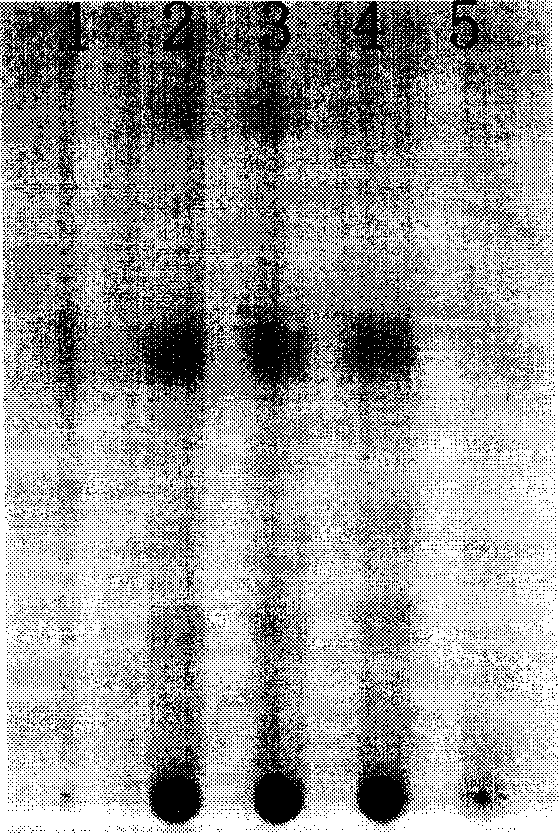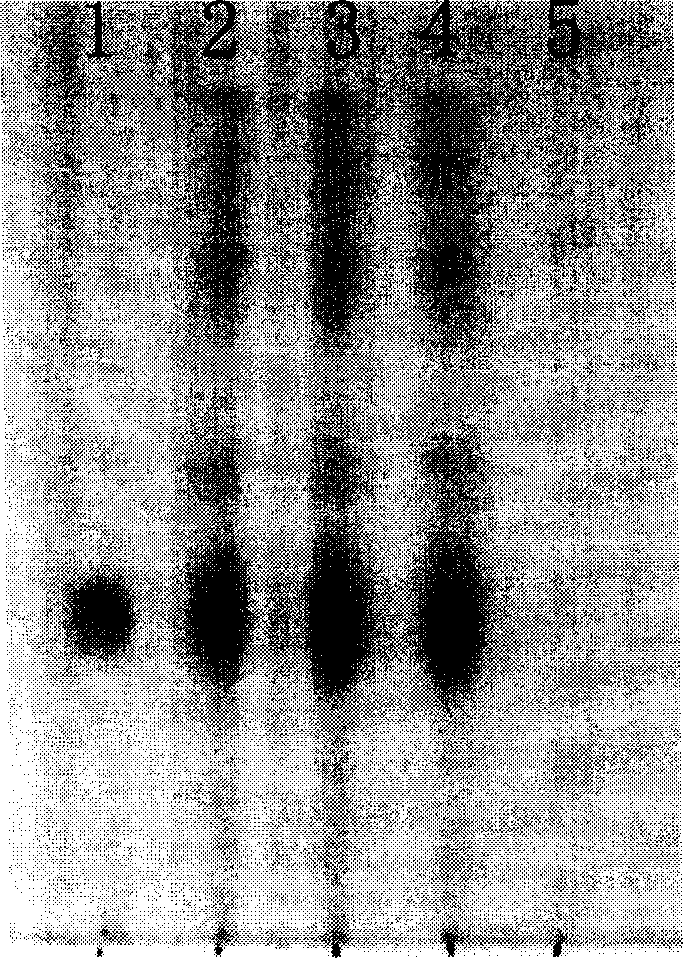Quality control method of kidney beneficial bone fortifying capsule
A quality control method and bone-building technology, applied in capsule delivery, medical preparations containing active ingredients, bone diseases, etc., can solve problems such as incomplete drug quality standards, difficulty in controlling drug quality, and lack of content detection methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] The preparation of embodiment 1 capsule
[0105] 【prescription】
[0106] Qiannianjian 180g Red Flower 60g Amomum Kernel 60g
[0107] Rehmannia glutinosa 180g Angelica 120g Polygonum multiflorum 180g
[0108] Salvia 120g Morinda officinalis 180g Eucommia 120g
[0109] Epimedium 240g Atractylodes macrocephala 120g Three seven 60g
[0110] Chuanxiong 120g Ginseng 60g Ligustrum lucidum 180g
[0111] Licorice 60g
[0112] [Preparation method] Ginseng is crushed into fine powder for the above sixteen medicinal materials; the other fifteen medicinal materials such as Polygonum multiflorum, add 8 times the amount of water and decoct three times, the first time for 3 hours, the second and third times for 2 hours each, and fry together liquid, filtered, and the filtrate was concentrated under reduced pressure to a clear paste with a relative density of 1.25 (measured at 80°C), mixed with the above-mentioned ginseng fine powder, dried, crushed into fine powder, added 3g of ma...
Embodiment 2
[0114] The quality control method of embodiment 1 capsule
[0115] 1), the character is observed, the method is as follows:
[0116] 【Properties】This product is a capsule, the content is brown to dark brown powder; slightly gas, bitter taste.
[0117] 2), identify the contents of angelica, chuanxiong, rehmannia glutinosa, salvia miltiorrhiza, licorice and ginseng, the method is as follows:
[0118] [Identification] 1. Take 4g of the content of this product, add 40ml of ether and 1ml of ammonia water, heat and reflux for 1 hour, take it out, let it cool, filter, evaporate the filtrate to nearly dry, add 0.5ml of ethyl acetate to dissolve the residue, and use it as a test product solution. Separately take 0.5 g each of Chinese angelica and Chuanxiong as reference medicinal materials, and make a solution of Chinese angelica and Chuanxiong as reference medicinal materials in the same way. According to the thin-layer chromatography ("Chinese Pharmacopoeia" 2005 edition one, appe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com