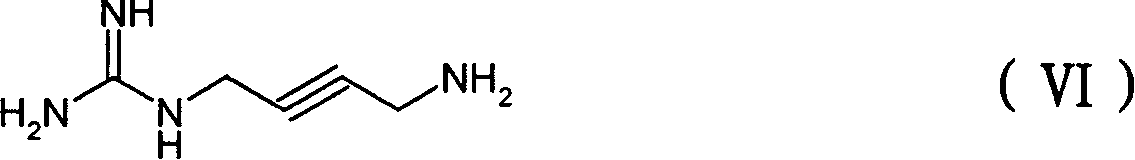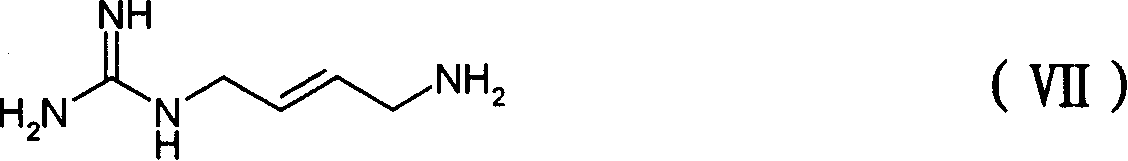Trilerium and trilerium marked agmatine and application in biochemistry and medicine research
An agmatine and labeling technology, which is applied in the field of agmatine compounds and its intermediates, can solve the problems of high cost, changes in physicochemical and pharmacological properties, and difficulty in the synthesis of labeled agmatine, and achieve simple synthesis methods and low cost. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1: [ 2 h 8 ] Preparation of Agmatine Sulfate:
[0078] 0.119g (1.23mmol) of [ 2 h 8 ]-1,4-Butanediamine and 0.171g (1.23mmol) of S-methylisothiouronium sulfate were dissolved in 15ml of water, reacted at 10-15°C for 5 hours, and then added dropwise sulfuric acid ethanol solution until the pH value was 6-7, the reaction solution was evaporated in vacuo, and the residual solid was recrystallized from 60% methanol to obtain 0.119 g of the title compound, with a yield of 41%.
[0079] 13 C-NMRδppm (D 2 O, DMSO): 158.26, 39.71, 39.50, 28.80, 25.83. MS (FAB) 139.2 (M+H).
Embodiment 2
[0080] Example 2: [ 3 h 4 ] Preparation of Agmatine Sulfate:
[0081] (1) Preparation of 4-amino-2-alkyne-butylguanidine sulfate
[0082] Dissolve 0.190g (2.26mmol) of 2-alkyne-1,4-butanediamine and 0.313g (2.26mmol) of S-methylisothiourea sulfate in 25ml of water, react at 10-15°C for 5 hours, then add dropwise The sulfuric acid ethanol solution was adjusted to a pH of 6-7, the reaction solution was evaporated in a vacuum rotary, and the residual solid was recrystallized with 60% methanol to obtain 0.174 g of 4-amino-2-yne-butylguanidine sulfate, with a yield of 34%.
[0083] 1 H-NMR δppm(D 2 O): 4.08(s, 2H), 3.85(s, 2H). 13 C-NMR δppm (D 2 O, DMSO): 158.35, 82.78, 77.18, 32.29, 30.67. MS(FAB)127.2(M+H), 225.2(M+H+H 2 SO 4 ). Elemental analysis (C 5 h 10 N 4 .H 2 SO 4 ): Calculated: C, 26.78%; H, 5.39%; N, 24.99%, found: C, 26.98%; H, 5.37%; N, 24.69%.
[0084] (2)[ 3 h 4 ] Preparation of Agmatine Sulfate
[0085] Dissolve 0.01g (0.045mmol) of 4-amino-2-yne...
Embodiment 3
[0086] Embodiment 3: [ 2 h 4 ] Preparation of Agmatine Sulfate:
[0087] Dissolve 0.01g (0.045mmol) of 4-amino-2-yne-butylguanidine sulfate in 3ml water, add 0.005g Pd / C catalyst (10%), and react with deuterium gas for 24 hours at normal temperature and pressure , Pd / C was filtered off, the reaction solution was concentrated to about 2ml, and 20ml of absolute ethanol was added to precipitate a solid, which was filtered to obtain the title compound.
[0088] 1 H-NMR δppm(D 2 O): 3.03(s, 2H), 2.82(s, 2H). 13 C-NMR δppm (D 2 O, DMSO): 158.26, 39.92, 39.71, 25.91, 25.44. MS (ESI) 134.2 (M).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com