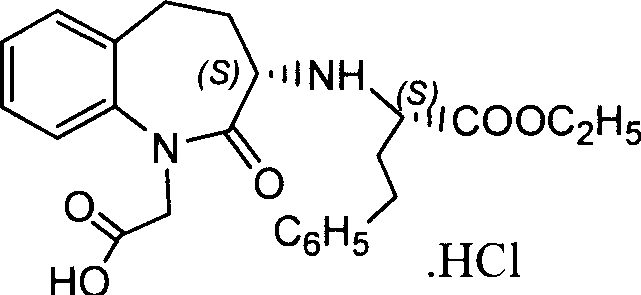
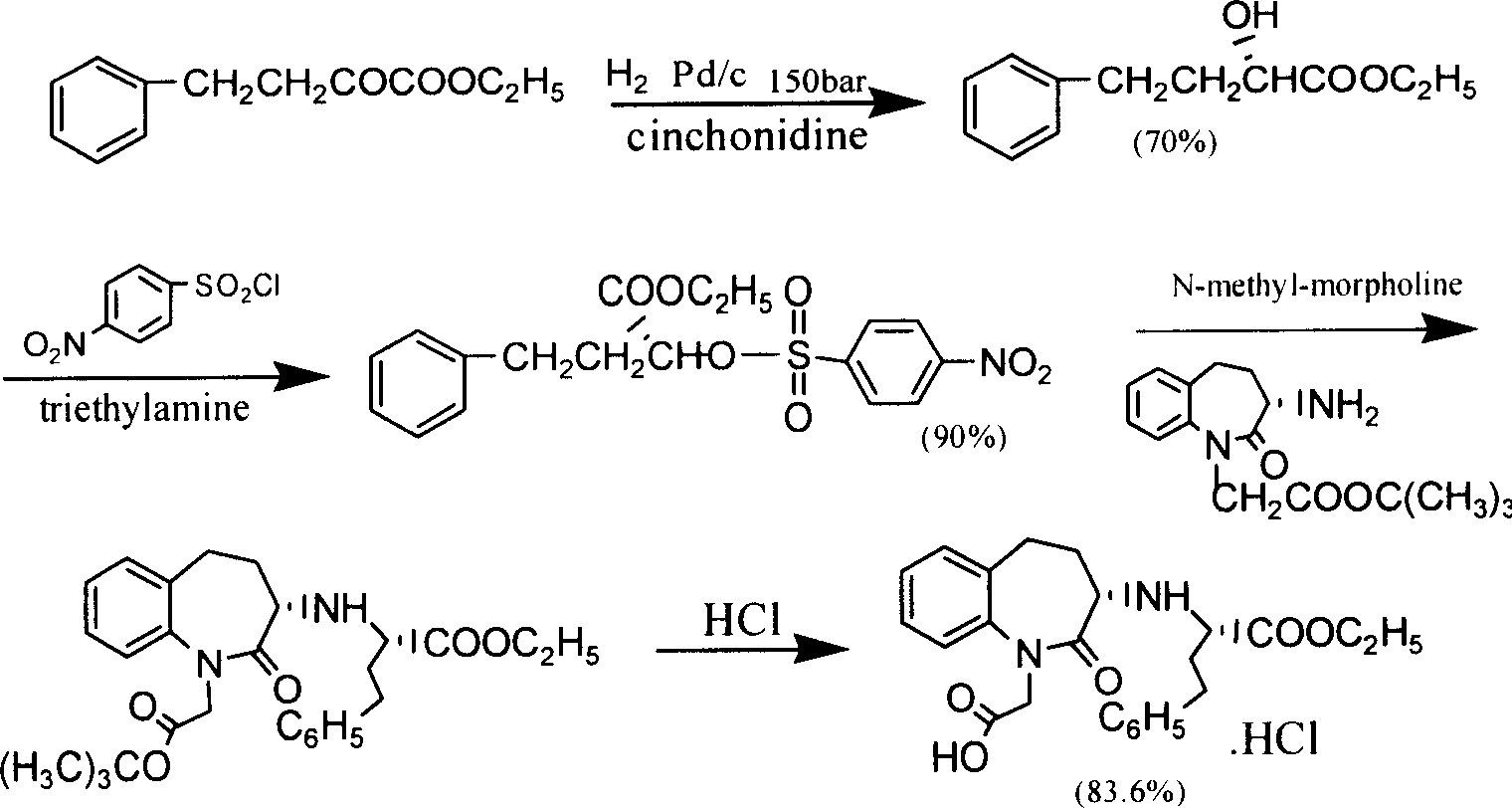
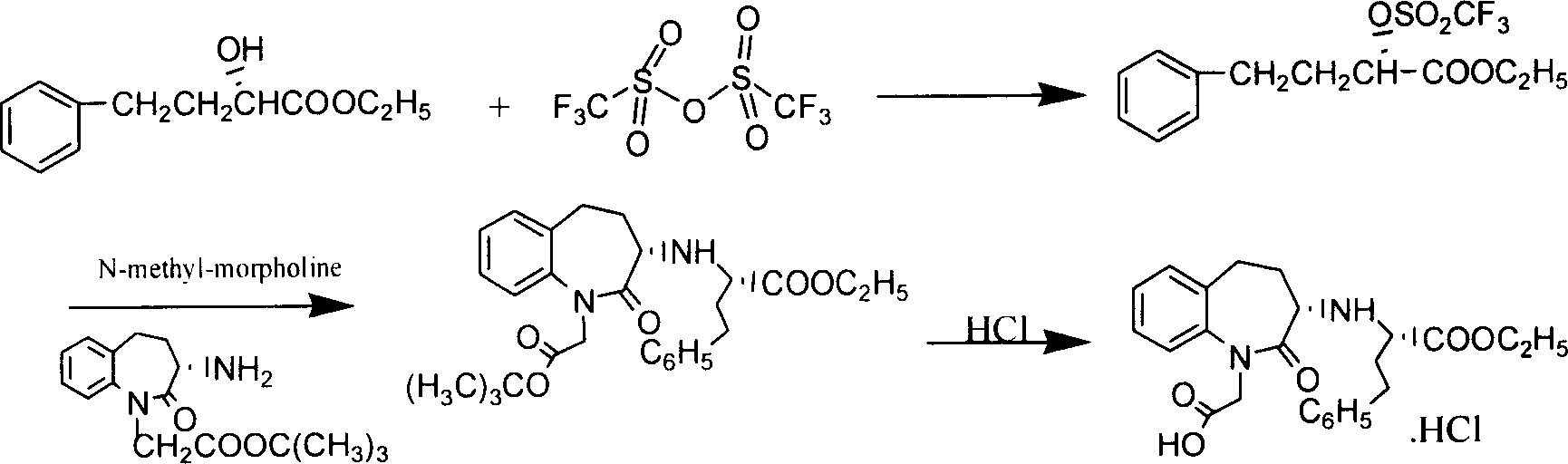
Process for preparing Benazepril hydrochloride materials
A technology of benazepril hydrochloride and acetic acid, applied in the field of medicine and chemical industry, can solve the problems of high cost of raw materials, difficult to purchase, etc., and achieve the effects of easy treatment of three wastes and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] 1.1 Preparation of 4,5-dihydro-3-phthalimide-1H-1-benzazepin-2-(3H)-one (II):
[0071] Raw materials / solvents
Feeding amount
Number of moles (mol)
The molar ratio of
Bromobenzocaprolactam I
8.00kg
33.32
1
Potassium phthalimide
7.30kg
39.42
1.18
32L
/
4(V / W)
Deionized water
80L
/
10(V / W)
[0072] Operation steps and process control:
[0073] Put 32L of N,N-dimethylformamide into the reaction kettle, put in 8.00kg of bromobenzocaprolactam (compound I) and 7.30kg of potassium phthalimide successively under stirring state, raise the temperature to 100-105°C, Maintain this temperature for 16 hours, cool the reaction solution to 20-30°C, slowly add 80L of deionized water dropwise, after the addition is complete, stir and crystallize at 5-10°C for at least 2 hours, filter, wash the filter cake with water, red...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com