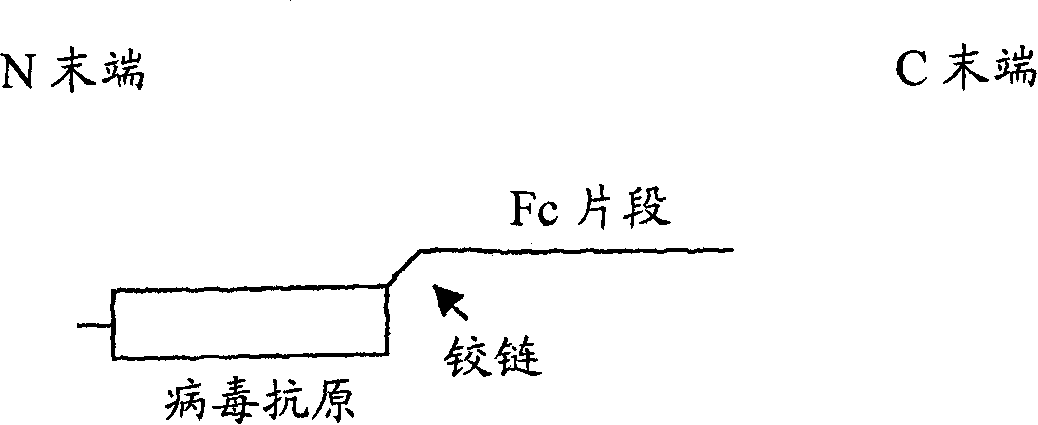
Chimeric antigens for breaking host tolerance to foreign antigens
一种嵌合抗原、抗原的技术,应用在使用载体引入外来遗传物质、融合多肽、抗细菌药等方向,能够解决没有有效预防性或治疗性疫苗等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] A. Example 1: Construction of TBD expression vector
[0150] Code C H 1-Hinge-C H 2-C H The murine IgGl DNA sequence of the amino acids of the 3-region portion was produced from mRNA isolated from a hybridoma (2C12), which produced a mAb directed against the HBV surface antigen (sAg). Using Trizol Reagents (Gibco BRL cat.No.15596-026) were used to isolate total mRNA and target binding domain (TBD) was generated by reverse transcription-PCR (RT-PCR) using Superscript First-strand Synthesis (Invitrogen Cat. ; mouse immunoglobulin fragment) cDNA. The PCR primers contained a linker sequence encoding a linker peptide - SRPQGGGS- (SEQ ID NO: 1) at the 5' end, a unique Not I site at the 5' end and a unique Hint III restriction site at the 3' end. Generated cDNA contains (5'NotI)-junction sequence-C H 1 (VDKKI) (SEQ ID NO: 2).-Hinge region-C H 2-C H 3-(3'Hind III). After digestion with the corresponding enzymes, this fragment was ligated with the pFastBac HTa expressi...
Embodiment 2
[0155] B. Example 2: Construction of Chimeric Antigen Expression Vector
[0156] DNA encoding the desired viral antigen was generated from the template using the PCR method using the 5' sense and 3' antisense primers in Table 2. The resulting amplified fragments contain a unique restriction site "5' enzyme" at the 5' end and a unique restriction site "3' enzyme" at the 3' end, each of which is used for ligation.
[0157] Table 2: Construction of chimeric antigen vectors
[0158] viral antigen
sense primer
antisense primer
template
5' enzyme
3' enzyme
HBV S1 / S2
SEQ ID NO: 7
SEQ ID NO: 8
pRSET BHV S1 / S2
Bam HI
Not I
HBV S1 / S2 / S
SEQ ID NO: 9
SEQ ID NO: 10
pAlt HBV 991
Nco I
Not I
HBV core
SEQ ID NO: 11
SEQ ID NO: 12
pAlt HBV 991
Nco I
Not I
DHBV PreS / S
SEQ ID NO: 5
SEQ ID NO: 13
pFastBac Hta PreS / S
Eco RI
Not I
DHBV PreS
SEQ ID NO: 5 ...
Embodiment 3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com