Heteronuclear compound with dicyandiamide connection and its medicinal use
A technology of heterocyclic compounds and cyanoguanidine, which is applied in the field of heterocyclic compounds composed of cyanoguanidine links and its pharmaceutical applications, can solve the problems of unsatisfactory preparation stability, unsatisfactory physical and chemical properties, and poor water solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
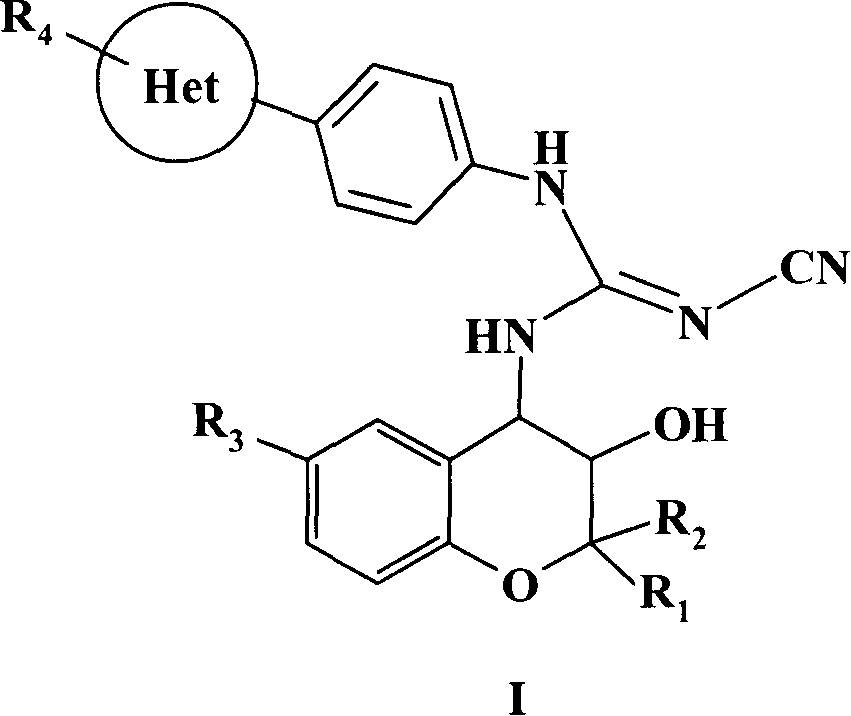
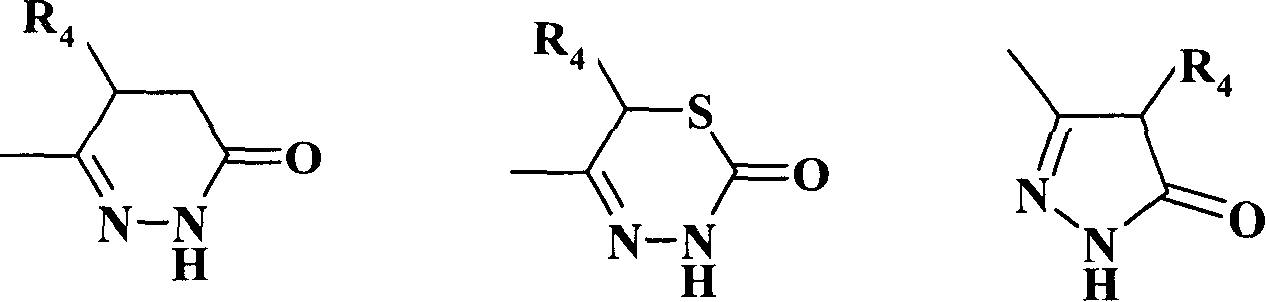
[0218] Example 1: (3S, 4R, 4'R)-N-[4-(4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl)phenyl] -N'-cyano-N"-(6-acetyl-3,4-dihydro-3-hydroxy-2,2-dimethyl-2H-1-benzopyran-4-yl)guanidine
[0219] 1.1 Synthesis of (4R)-4-(4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl)phenylthioisocyanate
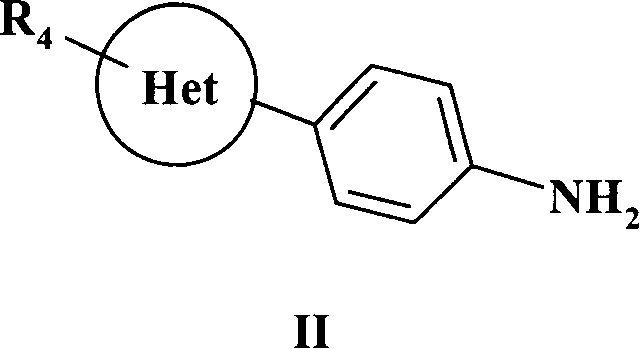
[0220] 3.45g (0.017mol) (5R)-(-)-6-(4-aminophenyl)-5-methyl-4,5-dihydropyridazin-3(2H)-one (2-1b) Soluble in 200ml 75% acetic acid and 100ml 2mol.L -1 In the mixed solvent of hydrochloric acid, under ice-water cooling, 500 ml of chloroform solution in which 2 ml (0.026 mol) of thiophosgene was dissolved was added dropwise thereto. The reaction was stirred at room temperature until the orange color of the chloroform layer disappeared, and the conversion of the raw material was detected by TLC. The organic layer was separated and washed with dilute NaHCO 3 aqueous solution and distilled water, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain (4R...
Embodiment 2
[0225] Example 2: (3R, 4S, 4'R)-N-[4-(4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl)phenyl] -N'-cyano-N"-(6-acetyl-3,4-dihydro-3-hydroxy-2,2-dimethyl-2H-1-benzopyran-4-yl)guanidine
[0226] The compound was prepared according to the method of 1.3 in Example 1, and the optically active 6-substituted benzopyran chiral amino alcohol was (3R, 4S)-4-amino-6-acetyl-3,4-dihydro-2 , 2-Dimethyl-2H-1-benzopyran-3-ol (1-3h'). 1 HNMR (600MHz, DMSO-d 6 , δ / ppm): 10.92(s, 1H), 9.48(s, 1H), 7.85-7.75(m, 5H), 7.38(d, J=7.8Hz, 2H), 6.85(d, J=8.4Hz, 1H), 5.89(broad peak, 1H), 4.96(m, 1H), 3.73(m, 1H), 3.39(m, 1H), 2.67(m, 1H), 2.50(s, 3H), 2.24(d, J=16.2Hz, 1H), 1.42(s, 3H), 1.19(s, 3H), 1.07(d, J=6.6Hz, 3H).
Embodiment 3
[0227] Example 3: (3S, 4R, 4'R)-N-[4-(4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl)phenyl] -N'-cyano-N"-{6-[(N,N-diisobutylamino)sulfonyl]-3,4-dihydro-3-hydroxyl-2,2-dimethyl-2H- 1-benzopyran-4-yl}guanidine
[0228] The compound was prepared according to the method of 1.3 in Example 1, and the optically active 6-substituted benzopyran chiral amino alcohol was (3S, 4R)-4-amino-6-[(N, N-diisobutylamino )sulfonyl]-3,4-dihydro-2,2-dimethyl-2H-1-chromen-3-ol (1-3a). 1 HNMR (600MHz, DMSO-d 6 , δ / ppm): 10.92(s, 1H), 9.55(s, 1H), 7.86(d, J=8.4Hz, 1H), 7.76(d, J=9.0Hz, 2H), 7.60(m, 1H) , 7.53(m, 1H), 7.37(m, 2H), 6.92(d, J=8.4Hz, 1H), 5.90(broad peak, 1H), 4.92(m, 1H), 3.78(m, 1H), 3.38 (m, 1H), 2.77(d, J=7.8Hz, 4H), 2.67(m, 1H), 2.24(d, J=16.2Hz, 1H), 1.85(m, 2H), 1.43(s, 3H) , 1.17 (s, 3H), 1.07 (d, J=7.2Hz, 3H), 0.84 (d, J=7.2Hz, 12H). MS (m / z, FAB): 638 (M+1) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com