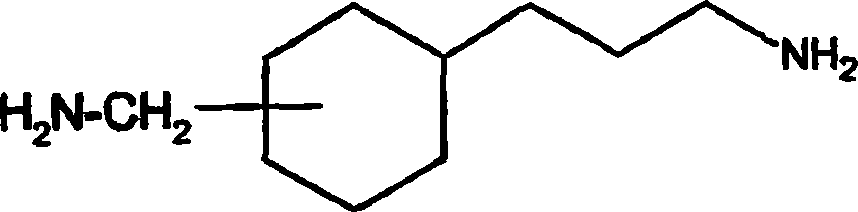
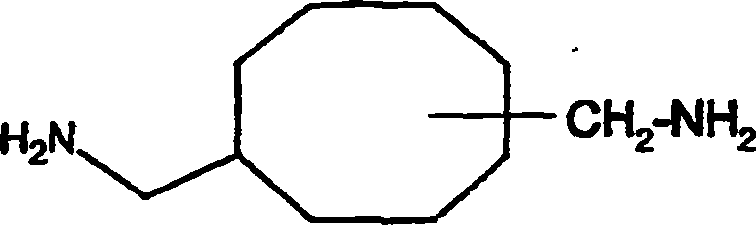
Thermohardenable epoxy resin-based compositions, 3(4)-(aminomethyl)-cyclohexane-propanamine and 1,4(5)-cyclooctane dimethanamine
A technology of epoxy resin and polyepoxide, applied in the direction of epoxy resin coating, coating, etc., can solve the problems of high yellowing tendency, high vapor pressure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0037] Recipe Components
[0038] With the reactive diamine C64 diamine and BAMCO, an epoxy system is obtained that is not brittle and cures well at room temperature.
[0039] Recipe Components
[0040] The use of C64 diamine and BAMCO, in combination with other amines, such as IPD, enables very reactive, non-tacky formulations with good performance.
[0041] Recipe Components
[0042] With this C64 diamine and BAMCO-epoxide system, the same excellent heat distortion resistance as in the case of cycloaliphatic diamines can be achieved, but the acetone resistance is improved.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com