Method for preparing alkane base containing phosphine bridge dichloropentate cyclooctane
A technology of alkane-based phosphine bridge and double spiro ring is applied in the field of preparation of organic compounds, which can solve the problems affecting the yield and quality of final products, restricting the large-scale promotion and application of products, etc., so as to improve the purity, effective components and yields The effect of high, high content of active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
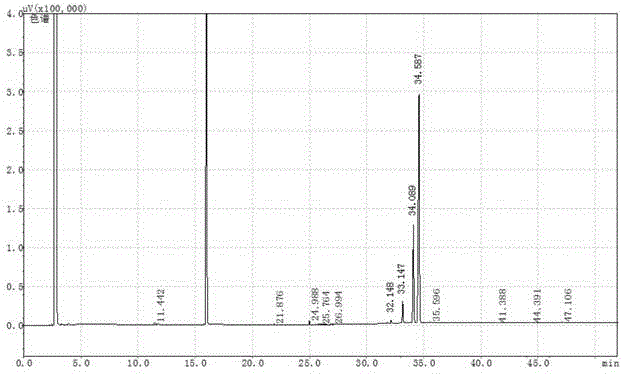
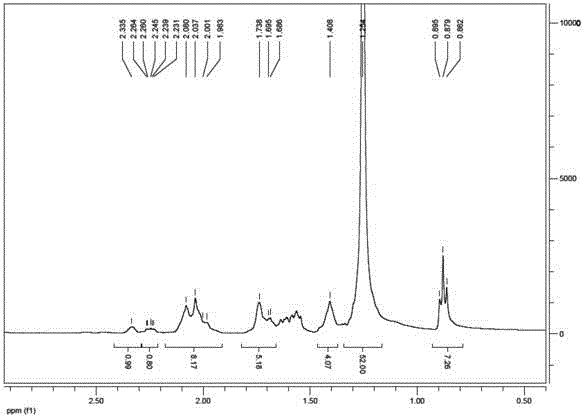
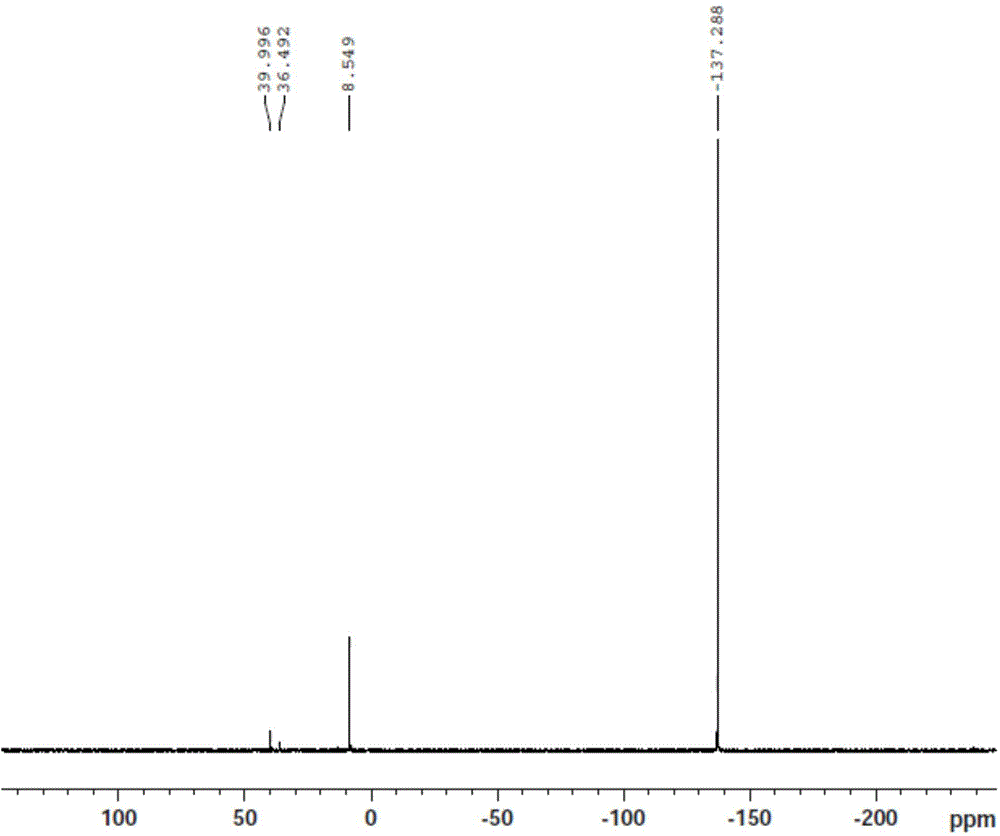
Image
Examples
preparation example Construction
[0027] According to one aspect of the present invention, there is provided a method for preparing alkane-containing phosphine bridged dispirocyclooctane, which includes the following three steps:
[0028] Step one, M 3 Preparation of P: In an ether solvent, add alkali metal M, elemental phosphorus and a naphthalene-based catalyst, where the alkali metal and elemental phosphorus react under the action of the naphthalene-based catalyst to obtain a mixture containing M 3 P's reaction system.
[0029] In the above step 1, the ether solvent is not particularly limited, as long as it can dissolve the material at a certain reaction temperature.
[0030] In the above step 1, the ether solvent is one ether solvent or any combination of multiple ether solvents.
[0031] As examples of the above-mentioned ether solvents, specifically mentioned: diethyl ether, n-butyl ether, diethyl ether, dipropyl ether, diisopropyl ether, dibutyl ether, dipentyl ether, diisoamyl ether, dihexyl ether, methyl ethy...
Embodiment 1
[0120] 1) Na 3 Preparation of P: Add 100ml of tetrahydrofuran, 6.9g of sodium metal, 3.1g of red phosphorus and 0.032g of naphthalene into the reaction vessel, and then react at 20°C for 5h;
[0121] 2) RPH 2 Preparation: Add 7.41g of tert-butanol and 10.90g of bromoethane to the reaction system obtained in step 1), and react at 20°C for 2 hours to obtain RPH 2 Reaction system, then saturated NH 4 The system was washed with Cl solution, after washing, it was extracted with n-hexane, and finally concentrated to obtain CH 3 CH 2 PH 2 ;
[0122] 3) Bridge: To the CH obtained in step 2) 3 CH 2 PH 2 Add 10.82g of 1,5-cyclooctadiene and 100ml of toluene, and react at 50℃ for 4h to obtain a reaction containing 9-ethyl-9-bridged phosphino-[3.3.1]-bisspirocyclooctane The system is concentrated and desolventized, and the final product containing 9-ethyl-9-bridged phosphino-[3.3.1]-bisspirocyclooctane is obtained by distillation. After calculation, the total yield of the final product is 42%....
Embodiment 2
[0125] 1) Na 3 Preparation of P: Add 40ml of 1,4-dioxane, 9.2g of sodium metal, 3.1g of white phosphorus and 0.15g of naphthalene into the reaction vessel, and then react at 30°C for 9h;
[0126] 2) RPH 2 Preparation: Add 14.82g of n-butanol and 24.6g of bromopropane to the reaction system obtained in step 1), and react at a temperature of 30°C for 3 hours to obtain RPH 2 Reaction system, then saturated NH 4 The system was washed with Cl solution, after washing, it was extracted with n-hexane, and finally concentrated to obtain CH 3 CH 2 CH 2 PH 2 ;
[0127] 3) Bridge: To the CH obtained in step 2) 3 CH 2 CH 2 PH 2 Add 21.64g of 1,5-cyclooctadiene and 100ml of toluene, and react at 0℃ for 19h to obtain a reaction containing 9-propyl-9-bridged phosphino-[3.3.1]-bisspirocyclooctane The system is concentrated and desolventized, and the final product containing 9-propyl-9-bridged phosphino-[3.3.1]-bisspirocyclooctane is obtained by distillation.
[0128] After calculation, the total yiel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com