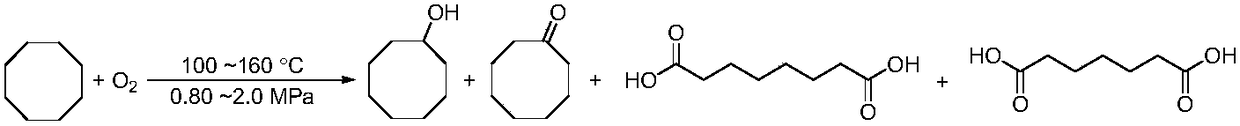
Novel catalyst-free oxidation method for cyclooctane
A cyclooctane and catalyst technology, which is applied in the field of cyclooctane catalyst-free oxidation, can solve problems such as environmental pollution, increased cyclooctane oxidation cost, unfavorable large-scale development of downstream products, etc., to achieve simple operation, prevent transition metal pollution, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] In a 100 mL stainless steel autoclave with a tetrafluoroethylene liner, feed 50.0 g of cyclooctane, and close the autoclave. Stir and slowly raise the temperature to 140°C, pass O 2 to 1.2MPa, at 140°C, 1.2MPa O 2 The reaction was stirred under pressure for 8.0 h, stirred and cooled to room temperature in an ice-water bath. The autoclave was turned on, and the obtained reaction mixture was filtered. The obtained solid was washed with 3×10 mL of cyclooctane, and dried in vacuum at 60° C., and the obtained white solid was analyzed by HPLC. The yield of suberic acid was 0.93%, and the yield of pimelic acid was 0.15%. Filtrate the obtained filtrate to rectify under reduced pressure, take 70 ℃ ~ 80 ℃ (20mm Hg) fraction, namely cyclooctanone, GC internal standard method analysis yield 5.68%; take 120 ℃ ~ 130 ℃ (22mm Hg) fraction, namely It is cyclooctyl alcohol, and the yield is 2.46% by GC internal standard analysis; at the same time, unreacted cyclooctane is recovered by ...
Embodiment 2
[0015] In a 100 mL stainless steel autoclave with a tetrafluoroethylene liner, feed 50.0 g of cyclooctane, and close the autoclave. Stir and slowly raise the temperature to 100°C, pass O 2 to 1.2MPa, at 100°C, 1.2MPa O 2 The reaction was stirred under pressure for 8.0 h, stirred and cooled to room temperature in an ice-water bath. The autoclave was turned on, the obtained reaction mixture was filtered, the obtained solid was washed with 3×10 mL cyclooctane, and dried in vacuum at 60° C., and the obtained white solid was analyzed by HPLC, and the yield of suberic acid was 0.0%, and the yield of pimelic acid was 0.0%. Filtrate the obtained filtrate to rectify under reduced pressure, take 70 ℃ ~ 80 ℃ (20mm Hg) fraction, namely cyclooctanone, GC internal standard method analysis yield 4.32%; take 120 ℃ ~ 130 ℃ (22mm Hg) fraction, namely It is cyclooctyl alcohol, and the yield is 0.04% by GC internal standard analysis; at the same time, rectification recovers unreacted cyclooctan...
Embodiment 3
[0017] In a 100 mL stainless steel autoclave with a tetrafluoroethylene liner, feed 50.0 g of cyclooctane, and close the autoclave. Stir and slowly raise the temperature to 160°C, pass O 2 to 1.2MPa, at 160°C, 1.2MPa O 2 The reaction was stirred under pressure for 6.0 h, stirred and cooled to room temperature in an ice-water bath. The autoclave was turned on, and the obtained reaction mixture was filtered. The obtained solid was washed with 3×10 mL of cyclooctane, and dried in vacuum at 60° C., and the obtained white solid was analyzed by HPLC. The yield of suberic acid was 1.85%, and the yield of pimelic acid was 0.87%. Filtrate the obtained filtrate and rectify under reduced pressure, take 70 ℃ ~ 80 ℃ (20mm Hg) fraction, namely cyclooctyl ketone, GC internal standard method analysis yield 6.58%; take 120 ℃ ~ 130 ℃ (22mm Hg) fraction, namely It is cyclooctyl alcohol, and the yield is 1.26% by GC internal standard analysis; at the same time, unreacted cyclooctane is recovere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com