Diagnostic marker for ovarian cancer
A technology of ovarian cancer and markers, which is applied in the field of cancer diagnosis and monitoring, and can solve problems such as diagnosis, grading or prognosis that do not prompt haptoglobin precursors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1 Removal of high-abundance albumin from human serum
[0081] Human serum samples were treated with a mixture of Affinity Gel-Blue and Protein A (5:1) in the form of a spin column (Bio-Rad Laboratories, USA). This spin column contains a mixture of Affinity Gel Blue and Protein A, which selectively binds and removes albumin and immunoglobulins. The spin column was washed twice with 1 ml of binding buffer (20 mM phosphate buffer, pH 7.0) by centrifugation at 1000 xg for 20 seconds. 50 μl of serum was added to 150 μl of binding buffer, mixed by vortexing, and loaded onto a spin column. After incubation for 1 h at room temperature, the column was centrifuged at 1000 x g for 20 sec to collect the eluate. The column was washed with 200 μl binding buffer and the first eluate was combined to generate a discarded serum sample. Determine the total protein concentration of the bound eluate. The eluate was stored at -80°C until further analysis.
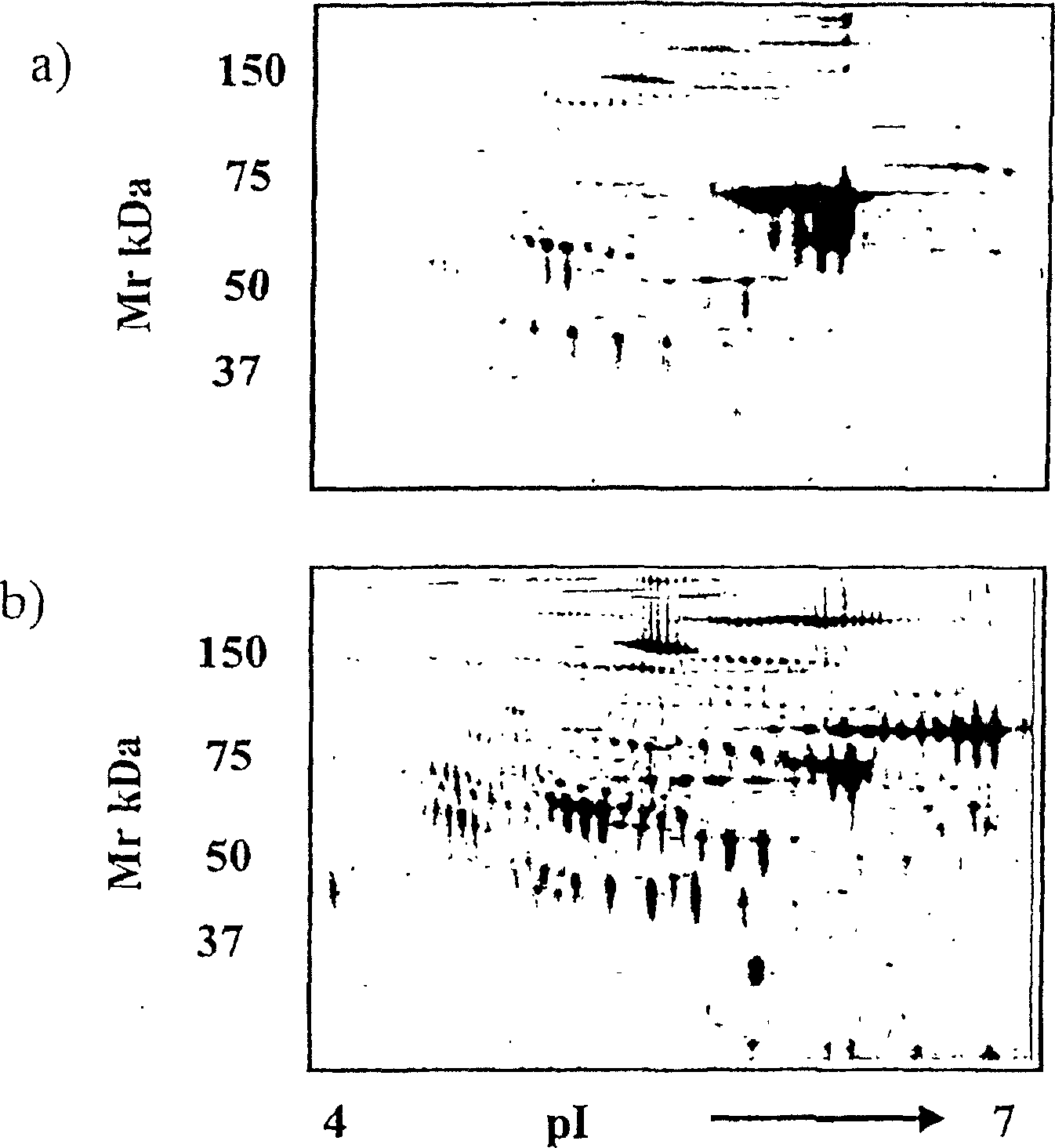
[0082] figure 1 a re...
Embodiment 2
[0083] Example 2 Expression of haptoglobin-1 precursor in serum and ascites of patients with ovarian cancer
[0084] Proteomic analysis and mass spectrometry were used to assess the expression of haptoglobin-1 precursor in the serum of normal healthy women and patients with ovarian cancer. Cancer patients were graded according to standard histological methods (Silverberg, 2000).
[0085] The mean ages of the women in the control group and women with ovarian cancer were 47 and 62 years, respectively. Whole blood (10 ml) was collected by venipuncture into flat collection tubes for serum (blood was allowed to clot for 30 minutes at room temperature). Samples were centrifuged at 2000 g for 10 minutes, followed by serum collection. Aliquots (100 [mu]l) were removed for determination of total protein. Serum was stored at -80°C until analysis.
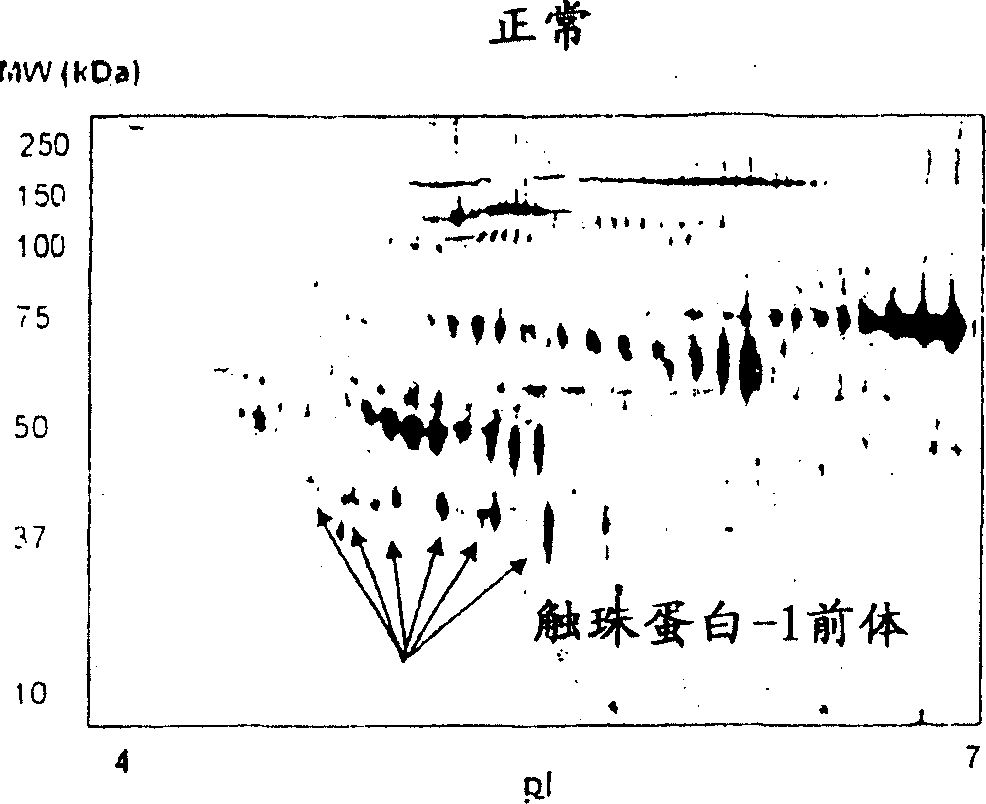
[0086] Serum samples from 8 normal female subjects and 19 ovarian cancer patients were analyzed for haptoglobin-1 precursor expression. ...
Embodiment 3
[0089] Example 3 Serum protein distribution of ovarian cancer patients with different histological grades
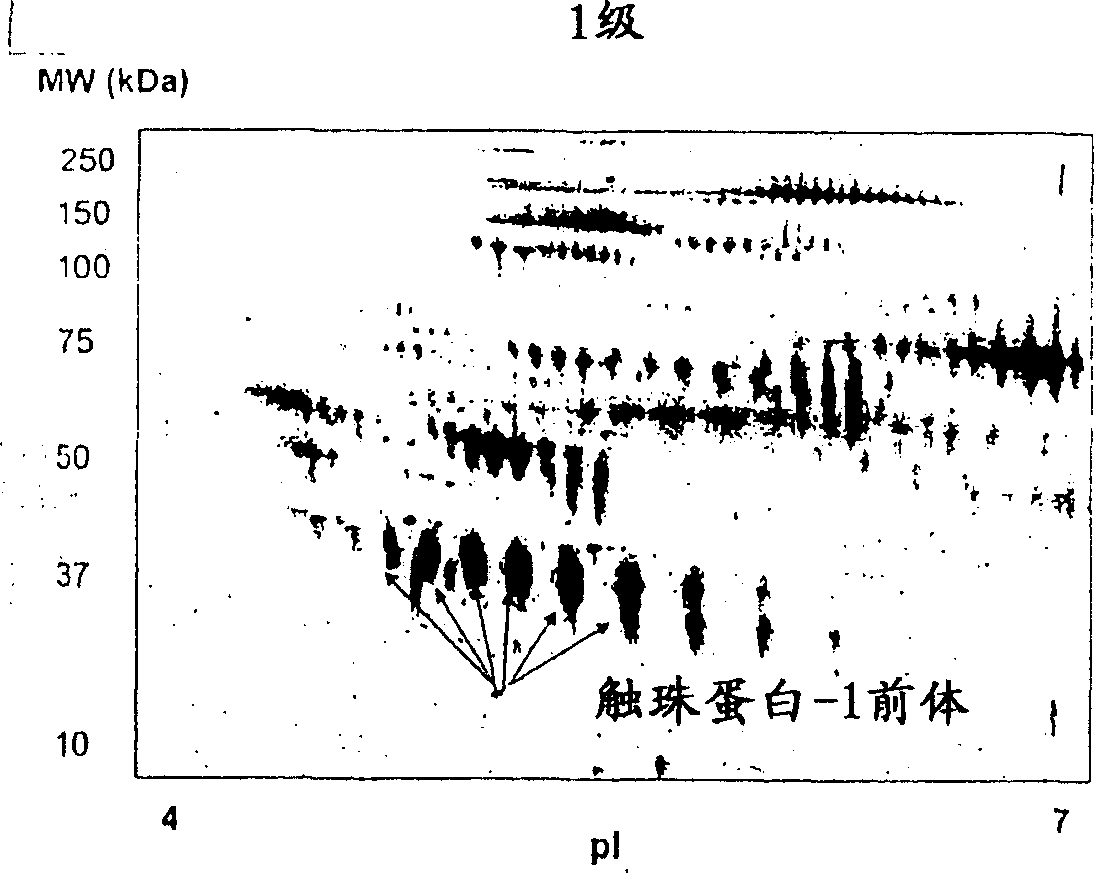
[0090] Protein profiles of grade 1 (n=6), grade 2 (n=8) and grade 3 (n=24) ovarian cancer patient sera were analyzed by 2-DE and visualized by staining with SYPRO-Ruby. Using PDQuest software to compare protein profiles from parallel groups of cancer patient samples and normal healthy women (n=8), and Figure 4 shown as a Gaussian distribution. Quantitative evaluation of serum proteins differentially expressed in normal group versus grade 1, 2, or 3 ovarian cancer patients was performed using Student's t-test. Significant differences in the total distribution of serum proteins were obtained in patients with grade 1, 2, and 3 ovarian cancer compared with normal healthy volunteers. Compared with normal serum, 24 proteins were differentially expressed in patients with grade 1 ovarian cancer ( Figure 4 a). Of these proteins, 15 proteins were upregulated 2-fold, 4 protei...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com